Emergent multidrug resistance
Antibiotic treatment is one of the main approaches of modern medicine which is used to prevent the spread of infections. From the 1930s to the 1960s, many new antibiotics were discovered which gave the period the name “golden era of antibiotics discovery”. Yet that era has come to an end due to the inability to maintain the pace of antibiotic discovery in the face of emergent resistant pathogens [1]. Antibiotic resistance poses a serious and growing global threat to humans and animals alike. Factors associated with the emergence of antibiotic resistance are the excessive use of antibiotics in humans and animals (e.g., livestock and fish farming), the release of nonmetabolized antibiotics into the environment through manure and feces as well as the growing human population, and the global migration. These factors increase selection pressure on bacteria resulting in the development of resistance through mutation or the acquisition of resistance genes and promoting the dissemination of resistant strains. Comprehensive efforts are needed to minimize the pace of resistance development by studying uncharacterized microorganisms and resistance mechanisms, and novel antimicrobial agents [2].
The Helmholtz Center for Infection Research
The HZI is a member of Germany’s largest scientific organization, the Helmholtz Association. In general, research at the HZI focuses on translational infection research including bacterial and viral pathogens, immune defense mechanisms, and novel active pharmaceutical agents, including antibiotics. The “microbial drugs” group led by Prof. Dr. Marc Stadler is investigating secondary metabolites i.e., biologically active natural products to derive potential antimicrobial agent candidates. Natural products are defined as chemical compounds produced by a living system. Only a few of these complex molecules can be produced by total synthesis in a cost-efficient manner [3]. Despite intensive global efforts, production by bacteria and fungi remains an efficient and successful strategy. Semisynthetic approaches modifying fermentation products can enhance product characteristics.
In the “microbial drugs” group at the HZI, natural products are mainly obtained from myxobacteria, actinobacteria, and filamentous fungi. The cultivation of such organisms is challenging due to the non-suspended growth. Filaments affect media viscosities and pellets have concentration gradients and mass transportation effects. In addition, complex media that often also contain solids are required to augment product titers compared to defined media [1, 4–5]. Many microorganisms have not been cultivated before and no standardized methods exist, making bioprocessing and subsequent scale-up a challenging endeavor.
Automation and digitalization
Prior to Lucullus®, bioprocesses were planned and managed as well as all process-associated information recorded in a Microsoft Access-based database for more than 20 years. Consumables such as media, feeds, supplements, and buffers were prepared according to an established Access-allocated printout protocol and various process-relevant information such as organism strains, cultivation conditions, and growth media composition were recorded in the database. Over the years, a huge amount of information and also errors have accumulated. With the update of the computer system software to Windows 10, the Access-based database was no longer compatible. Ever since data were managed manually.
In 2020, the “microbial drugs” department changed laboratories and a new technical facility was set up. With the challenging relocation, the timepoint was optimal for a technical leap into the future acquiring modern and new laboratory equipment and replacement of the outdated Access-based database. After the requirements for the new software were defined, encompassing process monitoring, process control, data storage capabilities, and additional planning and evaluation functionality, a comprehensive evaluation of four possible software solutions was conducted. With Lucullus®, the HZI found an ideal solution that fully met their requirements and even offered additional basic SCADA extended functionalities, and in Securecell AG a partner with profound and longtime experience in bioprocess automation and digitalization.
Lucullus® integrated platform technologies for candidate antibiotics production
The innovative bioprocessing laboratory at the HZI combines Lucullus® with several integrated modern multiple-scale fermenters including RAMOS® shake flasks, laboratory scale fermenters (DASGIP® bioreactors), small-scale fermenters (bbi-biotech), and technical scale bioreactors (Frings) to manage the demanding cultivations. All laboratory equipment including the fermenters and additional devices (scales, pumps) are connected over the local network to the Lucullus® database (Figure 1). From one single human-machine interface all process phases including process planning, process preparation, process execution, and most process evaluation steps are coordinated, implemented, and analyzed.
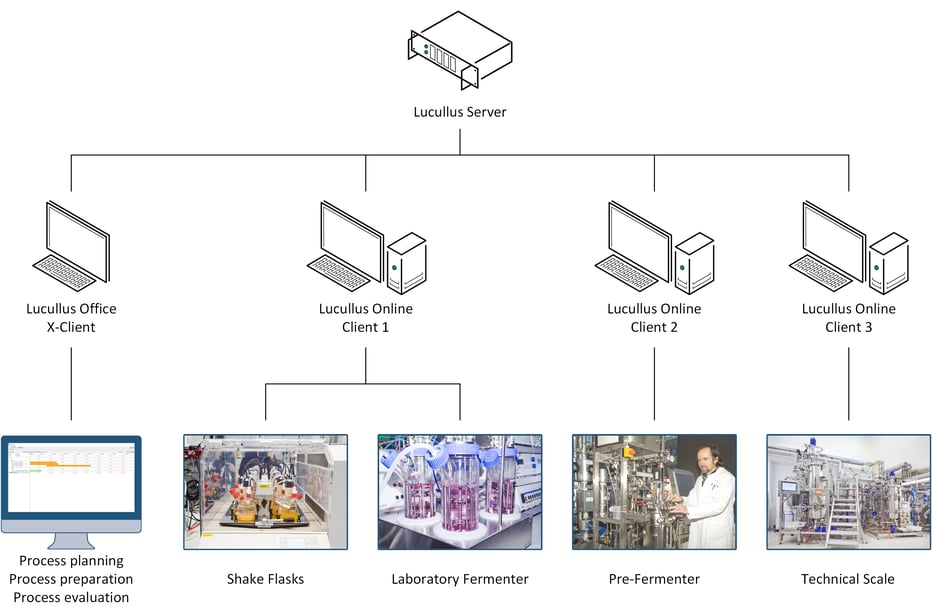
According to Miriam Grosse, head of the fermentation facility, one of the most valuable functions of Lucullus® is that all process data, including data from connected peripheral devices, are securely stored in real-time in a central database ensuring full data integrity and complete documentation. This allows the researchers to compare the data of running processes in the Lucullus® Graphics tool with data from historic processes to evaluate process performance and establish processes during upscaling (Figure 2). Moreover, with the Lucullus® Planning tool, future bioprocesses are precisely organized and configured with reactor utilization schedules, predefined process control operations, analytical plans, and media recipe selection. The integrated planning workflow at the HZI allows digitalized and efficient parallelization of experiments maximizing production. Instead of a printout protocol for media preparation, the corresponding recipes are now assigned to the process in the Planning tool. In the Lucullus® Media Kitchen tool, media management and preparation are digitally assisted with electronic protocols for media lot creation. With this unique feature, operator errors at the HZI are reduced and allow the convenient tracking of the raw material footprint in the final product.
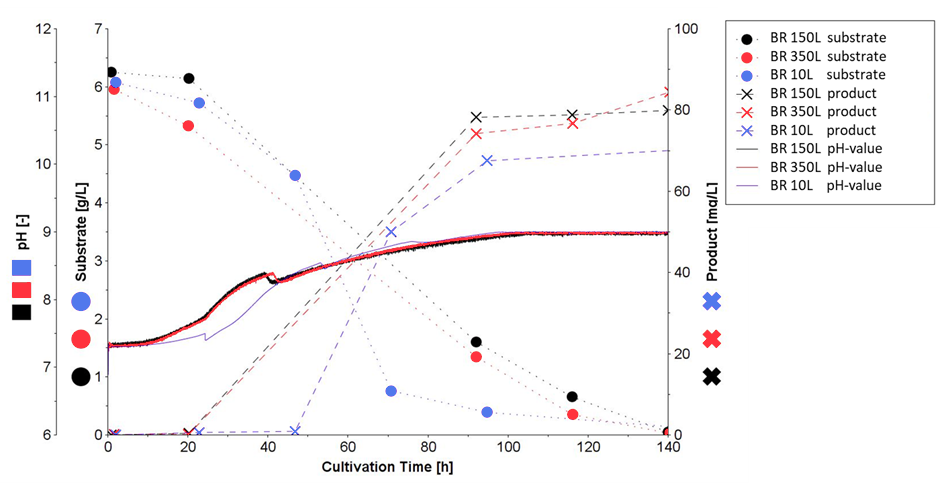 Figure 2: Process overlay showing the comparison of historic (10L and 150L) and currently running processes (350L).
Figure 2: Process overlay showing the comparison of historic (10L and 150L) and currently running processes (350L).
Summary
With Lucullus®, a modern laboratory environment at the HZI Braunschweig was digitalized and partly automated. The complex bioprocesses with cultivations of badly characterized microorganisms for the production of novel natural products to derive antimicrobial agent candidates are efficiently monitored and controlled with utmost data integrity. The potential natural product-derived drug candidates are produced in scale-up processes and evaluated for efficacy in pre-clinical studies with partners. The most appreciated function of Lucullus® at the HZI is that historic data are easily retrieved and can even be compared to currently running processes to swiftly react to changing process conditions of the pioneering cultivations.
Outlook
In the future, the HZI plans to expand the use of Lucullus® for automated centralized process control using programmable operations when processes are more established. In addition, optimized operations including attribute sets, setpoints, and control strategies for the routine processes will be established. Moreover, offline data management shall be implemented. To conclude, Lucullus® contributes to the establishment of difficult cultivation processes of microorganisms producing novel active agents and thus contributes to transforming therapies and new therapeutic options. In every aspect, this project serves as a textbook example. Both teams have been working hand in hand to reach a state-of-the-art laboratory environment.
References
-
Miethke M, Pieroni M, Weber T, Brönstrup M, Gilbert I, Stadler M, et al. (2021) Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem 5:726–749.
-
Aslam, B. et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist 11, 1645–1658 (2018).
-
Hanson, J. The classes of natural product and their isolation. in Natural Products: The Secondary Metabolites. Royal Society of Chemistry (ed. Hanson, J. R.) vol. 17 1–34 (The Royal Society of Chemistry, 2003).
-
Schiefer, A. et al. Corallopyronin A for short-course anti-wolbachial, macrofilaricidal treatment of filarial infections. PLoS Negl Trop Dis 14, e0008930- (2020).
-
Chaverra-Muñoz, L. & Hüttel, S. Optimization of the production process for the anticancer lead compound illudin M: process development in stirred tank bioreactors. Microb Cell Fact 21, 145 (2022).




