Information-driven future of the biomanufacturing industry
To improve global health, current and future medicines must become more effective and at the same time affordable. While healthcare systems around the world face rising costs, branded and generic drug manufacturers face the challenge of differentiating themselves in the market. The ongoing technological transformation and digitalization of the biomanufacturing industry give manufacturers the possibility to implement cutting-edge hardware and software solutions and thus an opportunity for differentiation from competitors.
Lucullus®, Securecell’s process information management system, is an example of such a cutting-edge software solution that empowers vendor-agnostic and overarching bioprocess monitoring and control. Lucullus® at its core is a SCADA (supervisory control and data acquisition) software that has been developed over the last 25 years together with longstanding biopharma customers. Over time, the general SCADA functionalities of Lucullus® have been extended with unique pre- and post-process functionality assisting operators along the upstream processing workflow.
Lucullus® at Teva Pharmaceuticals
According to Michael Thiele, head of the MSAT-USP and DSP departments at Teva in Baden-Württemberg, both regulatory and organizational reasons were the main drivers of why the department decided to implement Lucullus® as the software solution for their laboratory environment: “From a regulatory perspective, complete process documentation is important, including all events, interactions, and alarms before, during, and after a process. Such strict requirements for data integrity can only be met by vendor-agnostic and overarching process monitoring and control software. From an organizational perspective, workflows around bioprocesses are becoming more complex as the amount of data generated is increasing rapidly e.g., due to automated multi-bioreactor systems. Through comprehensive digitalization and integration of all process steps, the effort involved in servicing the bioreactors and in the final evaluation of the data can be substantially reduced. In both cases, this translates into more efficient and faster workflows requiring less operator interaction and thus, in the long run, saves time and money.”
Teva implemented Lucullus® at its full potential automating and digitalizing nearly all tasks along the upstream processing workflow.
The Planning tool
Teva uses the Lucullus® Planning tool to set up future bioprocesses and pre-define process details including reactor utilization schedules, process control operations, sampling and analytical plans including barcode label printing for off-line sample analysis, media recipe selection, and process genealogy. The genealogy function allows the linking of predecessor and successor bioprocesses to track quality-relevant parameters throughout the process chain.
The Media Kitchen tool
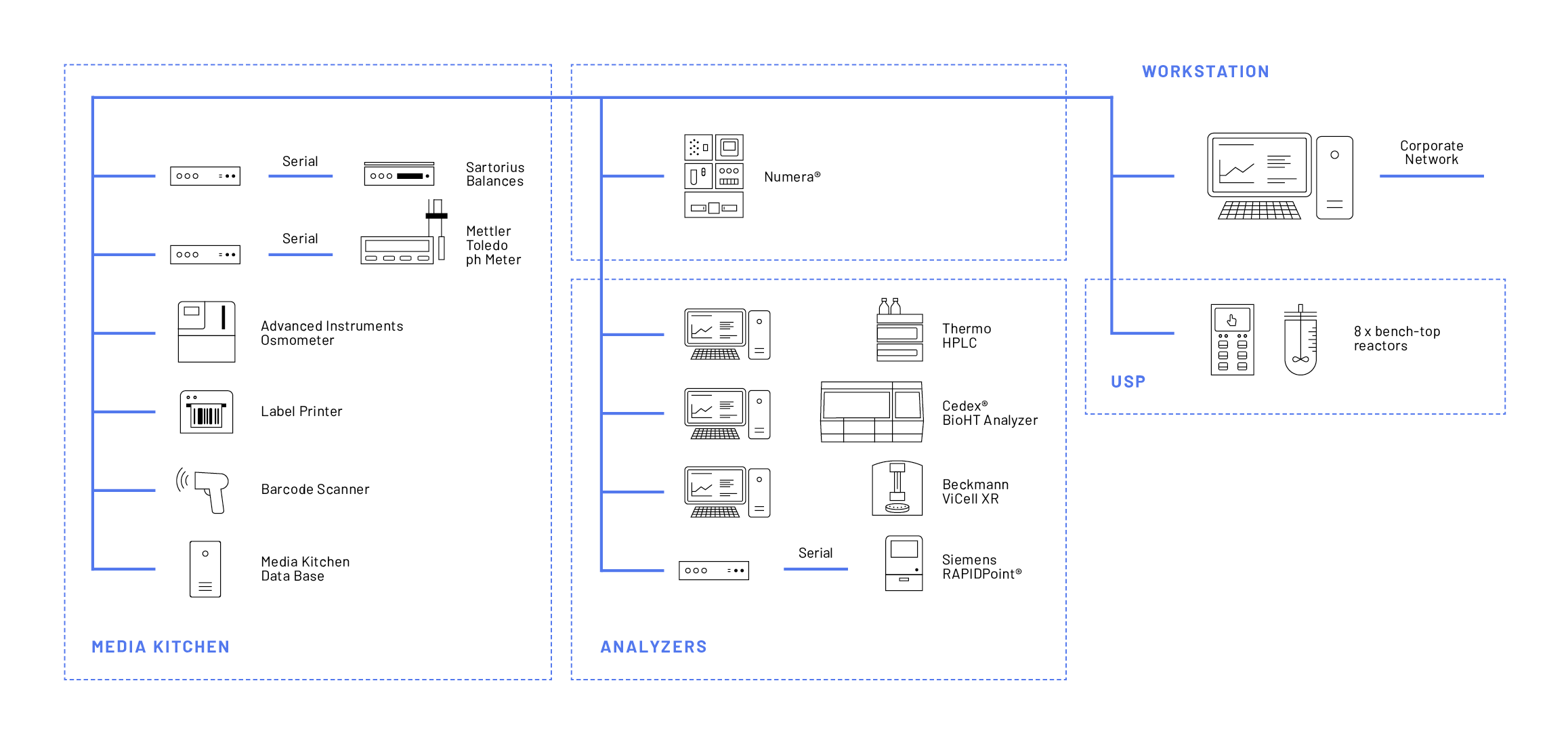
Teva employs the Lucullus® Media Kitchen tool for the preparation of all media and buffer lots. Digitally assisted raw material management and electronic protocols for media lot creation is a unique feature of the Lucullus® Media Kitchen tool, addressing the challenges of manual process preparation. Media and buffer solutions are prepared according to a guided procedure by barcode scanning described in more detail in Securecell’s application note #13, specifically dedicated to the Media Kitchen tool. At Teva’s MSAT-USP department, raw material and media management, and thus the entire process preparation phase, were digitalized with as few as 5 different devices (Figure 1).
The Online tool
The Lucullus® Online tool allows for user interactions, provides event-based notifications, and empowers the parallelization of processes. At Teva, currently, 8 reactors, 4 analytical devices, and the automated sampling system Numera® (besides the devices for the Media Kitchen) are integrated with Lucullus® for advanced feed-back control interactions (Figure 1). From one single human-machine interface, this extensive network of devices is effectively monitored and controlled.
The Graphic tool
In the Graphic tool, the Lucullus® data storage and evaluation center, process data of all historic and currently running processes are managed. The Graphic tool supports efficient database searches, extensive visualization, and automated evaluations. Furthermore, data export in many formats, as well as one-click batch reporting, is facilitated via the REST API interface. For Teva, this centralized and automated data storage greatly reduced manual efforts of data wrangling.
Comparison of former manual workflows to Lucullus® enabled workflows
During a parallel CHO fed-batch process in 8 bench-top reactors, different process parameters and metabolite levels e.g., pH, pCO2, pO2, cell viability, cell density, lactate, and glucose were recorded. The pH, pCO2 and pO2 were measured in-line while the cell viability, cell density, lactate, and glucose were measured off-line.
Manual vs. automated and digitalized bioprocessing
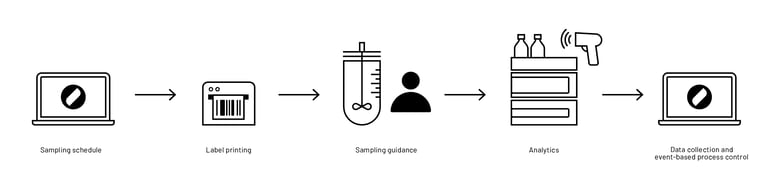
Before Lucullus® was introduced at Teva, the aforementioned off-line process parameters and metabolite levels were determined by manual reactor sampling and sample processing, followed by labeling of the tubes with a pencil and subsequent off-line analysis at the respective analyzer. The measurement values were then noted on paper or directly recorded in Excel and manually assigned to the respective process.
With Lucullus®, this highly manual workflow and the associated data management were transformed. The reactor sampling and sample processing are still done manually, however, the samples are stored in barcode-labeled tubes. With Lucullus® unique barcodes for each sample can be created. The sample barcodes are then scanned at the respective off-line analyzer. With the unique barcode ID generated by Lucullus®, the sample results are after the completed analysis automatically imported and assigned to the corresponding process and process time (Figure 2).
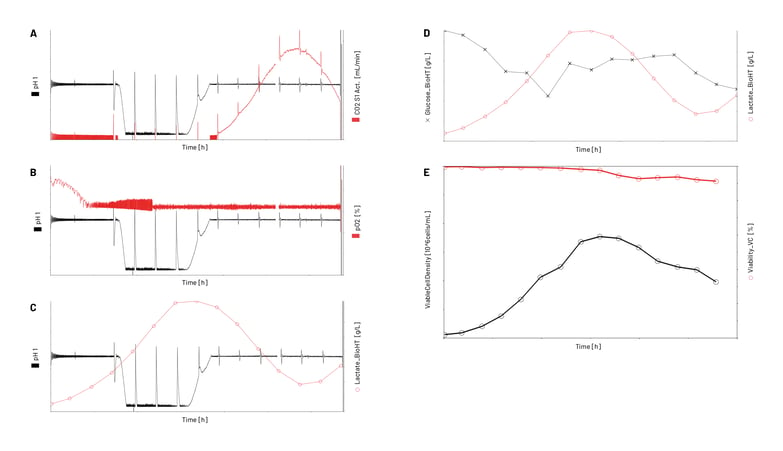
In Figure 3, the values of the measured process parameters or metabolite levels over the 14-day fed-batch process are shown. These results from one bioreactor exemplarily demonstrate the automated data import and assignment of analyzer data to the respective process for both in-line and off-line analyzer data. The near-real-time and automatically imported analyzer results can then be used as inputs for advanced preprogrammed feedback process control interactions
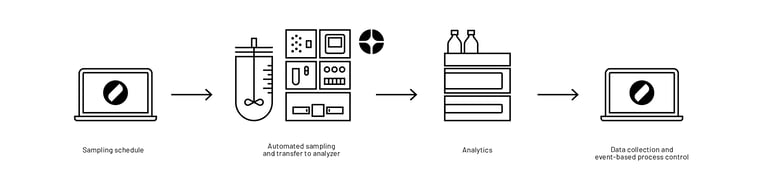
In combination with the automated sampling system Numera®, workflows at Teva were further automated (for a subset of reactors) by completely eliminating the need for manual bioreactor sampling, sample processing, and sample transfer (Figure 4). Numera® draws a sample from a connected reactor, processes it, and seamlessly transfers the sample to integrated analyzers for evaluation or to a sample storage unit. Thus, mostly all manual interactions around the bioprocess were eliminated at Teva by using both Lucullus ® and Numera®.
Conclusion
Lucullus® transformed the highly manual and labor-intensive upstream processing workflows at Teva into digital and automated ones. All interactions with the process and all changes to the data by operators can be tracked in the event log or audit trail. User errors due to sample exchange or potential mistakes during data transfer are vastly reduced, diminishing data wrangling and thus making processes more efficient and cost-effective. In combination with the automated sampling system Numera®, also the remaining manual tasks of sampling and sample processing at Teva were eliminated allowing for the implementation of automated and complex feedback control mechanisms.
Outlook
In 2023, the here presented Lucullus® installation in Teva’s MSAT facility will be expanded with new devices in a comprehensive client-server architecture. The expansion project will enable the complete digitalization of all steps in the USP process, from media production to data acquisition in the individual process stages and the production process. Securecell and Teva are looking forward to the integration project that supports an information-driven future in bioprocessing for safer and more affordable biopharmaceuticals.




