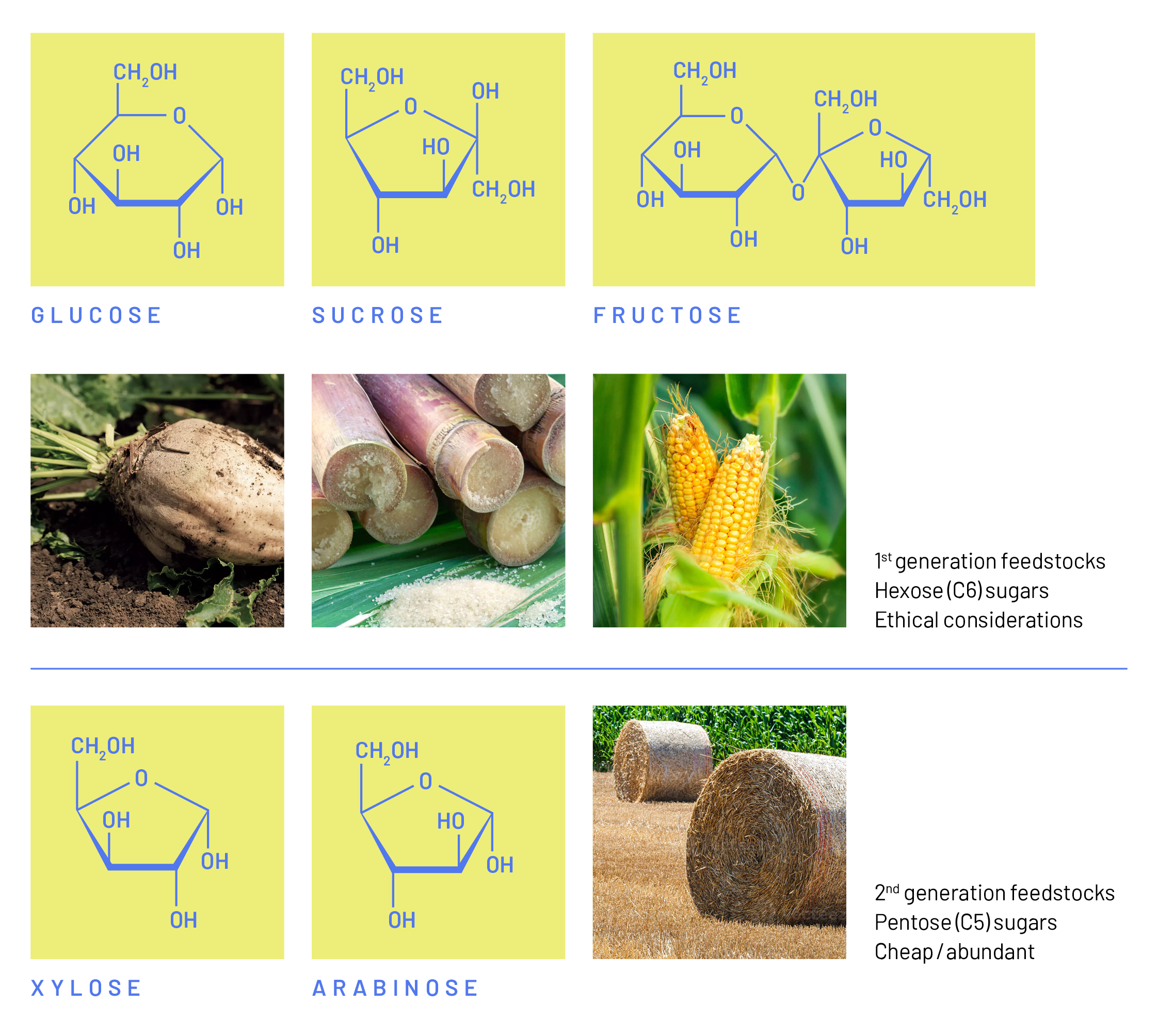
Production of Bioethanol
The growing demand for liquid transport fuels alongside concerns about climate change caused by greenhouse gas emissions from the use of fossil fuels has become one of the greatest challenges for modern society. The generation of biofuels from biomass is a sustainable solution that could substantially decrease the usage of fossil fuels (Herrera, 2006). The yeast, S. cerevisiae, is an excellent host-microbe and a widely used cell factory for the biomass-based production of fuels and chemicals, in particular ethanol. Common substrates that S. cerevisiae uses in the industrial production of ethanol are hexose sugars such as sucrose, glucose, or fructose (Figure 1). These substrates are derived mostly from sugar beets in Europe, sugar cane in South America, or corn in India and the United States (Kang & Lee, 2015). As these so called first-generation feedstocks have the potential to serve as a valuable food source and influence deforestation and biodiversity, ethical considerations arise when utilizing them for the production of ethanol (Paravantis, 2022). There is substantial interest in switching to alternative feedstocks such as waste streams from agricultural production, which are considered second-generation feedstocks. Similar to first-generation feedstocks, second-generation feedstocks are relatively rich in hexose sugars. In addition, they also contain significant amounts of pentose sugars, such as xylose and arabinose. The production of ethanol from these pentose sugars would be interesting from both a social and an economic perspective. Unfortunately, when S. cerevisiae is exposed to hexose and pentose sugars during cultivation, the yeast readily uses the hexoses, but only slowly consumes the pentose sugars. In order to cost-effectively produce biofuels from plant biomass, all sugars, including all pentose and hexose sugars present in the raw starting material must be converted efficiently into the final products.
Generating a yeast strain that efficiently uses pentose and hexose sugars has been an object of extensive research for several decades. In a study employing an evolutionary engineering approach, promising results were achieved. An engineered S. cerevisiae strain capable of metabolizing pentose sugars was used to explore whether an evolutionary engineering approach could lead to the development of enhanced strains with improved pentose sugar consumption. Before the evolutionary experiment, the strain had a generation time of multiple days. This strain was inoculated in shake flasks containing medium where only pentose sugars were supplied as a carbon source. Whenever the culture reached a high cell density, part of the culture was transferred into a new shake flask. After repeating this growth and transfer process many times, eventually, an evolved strain was obtained with a generation time of less than 1 day. Whole-genome sequencing was performed of the evolved strain and a single amino acid mutation was detected in a hexose transporter. The evolved strain showed improved xylose uptake rates, as well as partial co-utilization of glucose and xylose in mixed sugar cultivations (Reider Apel et al., 2016). The capability of this strain to consume both glucose and xylose at comparable rates is a very relevant characteristic to efficiently and industrially produce ethanol from second-generation feedstocks.
“Using the Lucullus® software, we are able to cultivate microbial cultures for long periods with complex feeding routines. To ensure optimal control of culture parameters and minimize manual labor, it is essential to have reliable and adaptable culture automation software. Our experience with the Lucullus® software has been very positive, and we have received excellent support in customizing its features to fit our needs.”

Automated Evolutionary Engineering Experiments
In the previous example, cells were cultivated in shake flasks. For the automation of evolutionary engineering experiments, cultivations in bioreactor systems are better suited, as the local environment can be controlled much more precisely and therefore, the process conditions can be better defined and control tasks automated. As the setup of a bioreactor experiment is more complex than the setup of a shake flask experiment, a protocol was written that outlines a generic setup for automated evolutionary experiments using Getinge Applikon bioreactor systems combined with the process information management software Lucullus® (de Hulster et al., 2022).
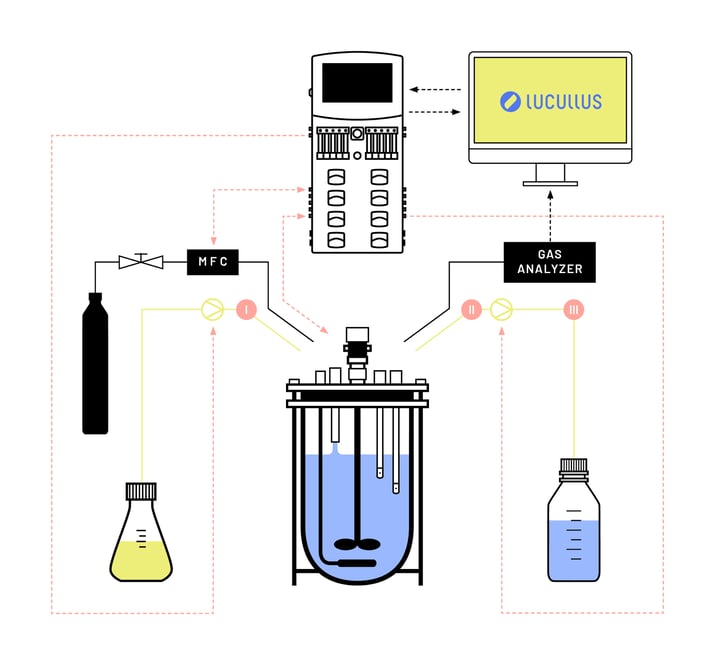
The material requirements for evolutionary engineering experiments vary depending on the target strain, desired evolutionary outcome, and selective conditions in the culture. Figure 2 shows a schematic representation of the bioreactor setup that was used for the automated evolutionary engineering experiments with aerobic and anaerobic cultures of S. cerevisiae with a variety of carbon sources as growth-limiting nutrients. The setup consisted of (1) an autoclavable bioreactor (2) an electronic liquid level sensor, (3) a medium inlet, (4) an effluent pipe near the reactor bottom, (5) a medium reservoir connected to the bioreactor with a controllable pump, (6) a waste container connected to the bioreactor with a controllable pump, (7) a pressurized gas line with mass flow controller (MFC), and (8) a the Lucullus® control software for the automation of the setup during the evolutionary engineering experiment.
Sequential Batch Cultures
Different cultivation strategies for evolutionary engineering experiments exist, namely sequential batch cultivation, chemostat cultivation, and accelerostat cultivation. The different cultivation strategies are described in detail in the following paper: Under pressure evolutionary engineering of Yeast Strains for improved performance in fuels and chemicals productions (Mans et al., 2018). In this article, the concept of evolutionary engineering is described for sequential batch cultivations (repeated batch).
The operator starts the preparation of the materials for the automated evolutionary engineering experiment (Figure 2). The prepared bioreactor is inoculated with the first batch. This first batch is normally not representative in terms of batch time and other process values (Figure 3A). After the first, non-representative batch, the emptying and refilling of the reactor are performed manually. The reactor is then inoculated with the second batch. After the second batch, the batch time is determined (20 h), the highest outflow CO2 value (blue line; an indicator of the number of cells in the bioreactor) is measured (0.46%) and the cumulative level of CO2 (orange line; an arbitrary value estimating how much of the carbon substrate was consumed) is calculated (3.1) (Figure 3B). These three process values are used to set the threshold parameters for the automated empty and refill cycles in the Lucullus® software. To safeguard against unintended emptying/refill cycles, checkpoints of batch time, outflow CO2 level decrease, and cumulative level of CO2 are set (Figure 3C).
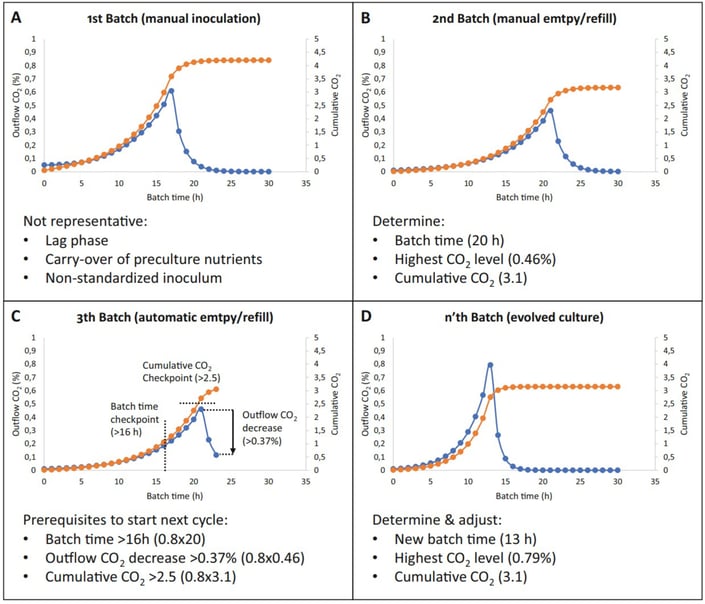
The prevention of unintended empty/refill cycles is important because the loss of a significant part of the population might perturb the automated evolution. After these threshold values are set in the software Lucullus®, the sequential batch process can run on its own. Over time, the culture starts evolving i.e., the batch time, highest outflow CO2 value, and cumulative level of CO2 change (Figure 3D). Periodically these threshold parameters need to be adapted to make sure that the empty/refill cycles are still triggered at the right time, which is fundamental to keep the culture evolving.
Software Lucullus®
The Lucullus® process information management system is a comprehensive bioprocess automation software. Besides basic SCADA functionalities that allow for device monitoring and control of over 100 devices found in typical bioprocessing environments, Lucullus® has an inbuilt sample management and raw material management functionality, and interfacing capability with various LIMS and ELN systems, as well as statistical software packages, such as Phyton, MATLAB, and DataHowLab.
Sequential Batch Cultures with Lucullus®
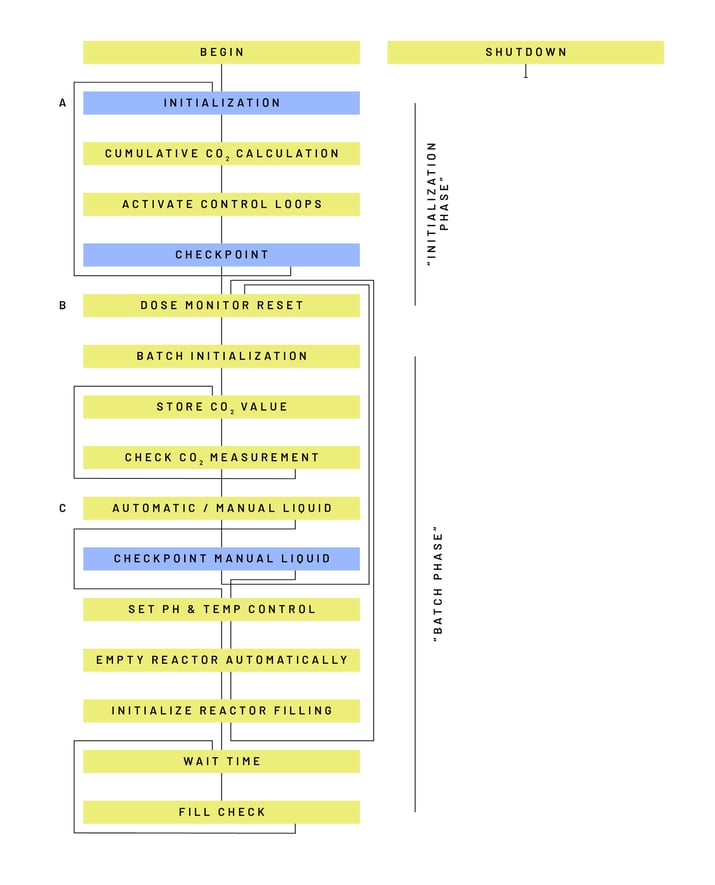
Researchers at the TU Delft used Lucullus® to automate and digitalize their evolutionary engineering experiments. Figure 4 shows a schematic of the Operation or Step Chain/process recipe used to automate the Repeated Batch experiment. The Operation guides the operator through the setup of the process with manual user interaction steps (blue blocks) and thereafter executes the bulk of the process autonomously (yellow blocks).
Explanation of the Repeated Batch Operation
The individual steps of the Step Chain presented in Figure 4 are comprised of the
following functions:
- Begin: Default step with no commands, executed at the start of every process
- Initialization: User interaction step, checking/adjusting critical process parameter values
- Cumulative CO2 Calculation: Formula definition step, calculating the cumulative amount of CO2 produced in the bioreactor
- Activate Control Loops: Controller activation step, starting pH, stirrer, and temperature control
- Checkpoint: User interaction step, checking whether to proceed to the Dose Monitor Reset step or return to the Initialization step
- Dose Monitor Reset: Step, resetting the totalizer values of the gas flow, acid, and base pumps
- Batch Initialization: Step, starting (the first or one of the consecutive) batch cultivations
- Store CO2 Value: Step, storing the value of the CO2 off-gas measurement for subsequent value comparison (see Check CO2 Measurements step)
- Check CO2 Measurements: Step, comparing the current CO2 value with the previously stored value. Loops back repeatedly until the end of the batch is reached
- Automatic / Manual Liquid Cycling: Step, directing Lucullus® to an automated or manually executed medium exchange
- Checkpoint Manual Liquid Cycling: step, allows the user to confirm the completion of a manual medium exchange or to switch to an automated medium exchange
- pH & Temp Control: pH and temperature control step, activating or deactivating pH and temperature control loops as selected by the user during the initialization
- Empty Reactor Automatically: Harvest step, emptying the reactor automatically by the harvest pump, based on a predefined pump time activity selected by the user during the initialization
- Initialize Reactor Filling: Refilling step, adding fresh medium to the reactor by the feed pump
- Wait Time: Waiting step of 6 seconds that is required for the Fill Check step
- Fill Check: Step, checking the level inside the bioreactor. May loop back to the previous step repeatedly.
An example of a manual user interaction step from the Repeated Batch Operation (Figure 4) is shown in Figure 5. A pop-up window signifies the manual interaction step. Within this window, the operator encounters a list of parameters labeled as „Name“. Each parameter can be associated with a scientific unit (“Unit”). To assist the operator, a description can be provided for each parameter (“Description”), as well as an acceptable range of values (“Range”). The default process values assigned to each parameter can be modified before starting the process (“Default”).
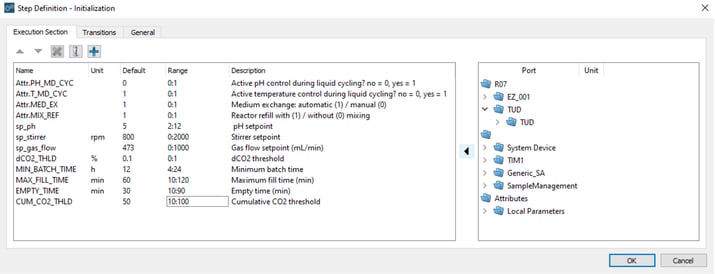 Figure 5: Example of the “Initialization” manual interaction step in the Repeated Batch Operation.
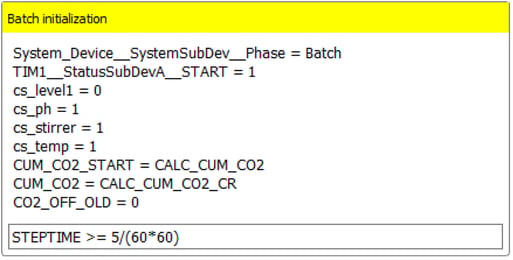
Figure 5: Example of the “Initialization” manual interaction step in the Repeated Batch Operation.During the process, Lucullus® signals the current execution stage through process indicators known as “Phases”. In Figure 6, an excerpt of the “Batch Initialization” step of the Repeated Batch Operation is shown, marking the start of the batch phase. Once the batch phase has reached a point where the criteria for termination are fulfilled (see “Check CO2 Measurements” step), Lucullus ® will then proceed with the “Empty Reactor Automatically” step, provided that the user has not opted before for manual medium exchange (Figure 4). The “Empty Reactor Automatically” step is succeeded by the “Fill Reactor Automatically” step, after which a subsequent batch phase is started.
A crucial part of the Repeated Batch Operation is the monitoring CO2 in the off-gas. By programming a loop consisting of two interconnected steps, Lucullus® keeps track of the highest recorded value of CO2 for a particular batch. The two-step loop is presented in Figure 7.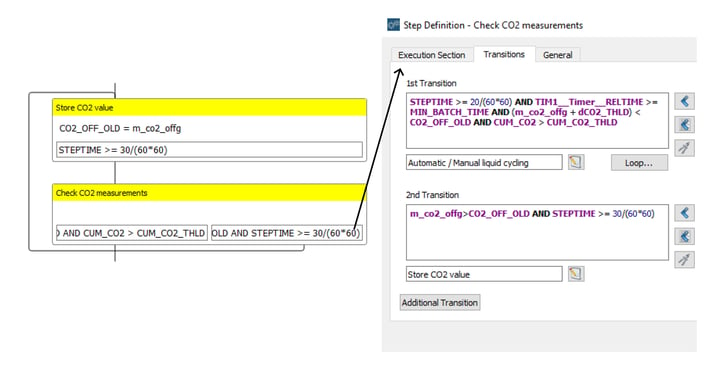
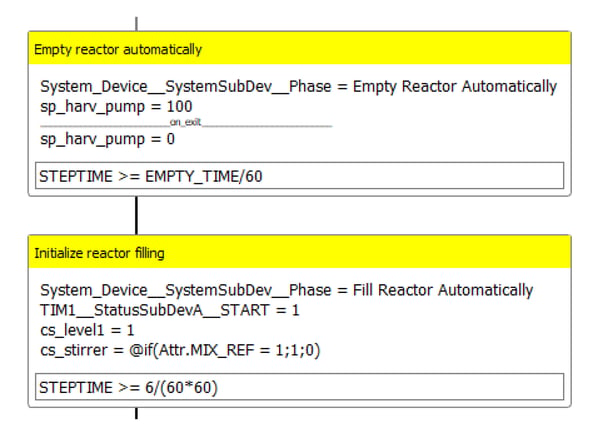
When a batch has been completed, the majority of the fermentation broth needs to be removed (harvested). A small volume of liquid that remains in the bioreactor after harvesting serves as inoculum for the next batch. Operators can select the empty and refill of the bioreactor manually or automatically. In the second case, Lucullus® is programmed to perform the harvest and refill by executing two consecutive steps (Figure 8).
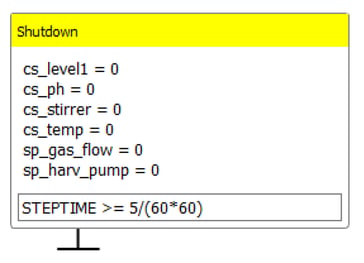
Lucullus® automatically ceases all active control loops and actuators upon the conclusion of an experiment. This functionality is illustrated in Figure 9.
Related Webinar
This article was showcased in a webinar. To view the recorded version of this webinar, please follow this link:
References
-
(de Hulster, E., Mooiman, C., Timmermans, R., & Mans, R. (2022). Automated Evolutionary Engineering of Yeasts. In V. Mapelli & M. Bettiga (Eds.), Yeast Metabolic Engineering: Methods and Protocols (pp. 255–270). Springer US.https://doi.org/10.1007/978-1-0716-2399-2_15
-
Dragosits, M., & Mattanovich, D. (2013). Adaptive laboratory evolution – principles and applications for biotechnology. Microbial Cell Factories, 12(1), 64. https://doi.org/10.1186/1475-2859-12-64
-
Herrera, S. (2006). Bonkers about biofuels. Nature Biotechnology, 24(7), 755–760. https://doi.org/10.1038/nbt0706-755
-
Kang, A., & Lee, T. S. (2015). Converting Sugars to Biofuels: Ethanol and Beyond. Bioengineering, 2(4), 184–203. https://doi.org/10.3390/bioengineering2040184
-
Mans, R., Daran, J.-M. G., & Pronk, J. T. (2018). Under pressure: evolutionary engineering of yeast strains for improved performance in fuels and chemicals production. Current Opinion in Biotechnology, 50, 47–56. https://doi.org/https://doi.org/10.1016/j.copbio.2017.10.011
-
Paravantis, J. A. (2022). 38 - Socioeconomic aspects of third-generation biofuels. In E. Jacob-Lopes, L. Q. Zepka, I. A. Severo, & M. M. Maroneze (Eds.), 3rd Generation Biofuels (pp. 869–917). Woodhead Publishing.https://doi.org/https://doi.org/10.1016/B978-0-323-90971-6.00046-2
-
Reider Apel, A., Ouellet, M., Szmidt-Middleton, H., Keasling, J. D., & Mukhopadhyay, A. (2016). Evolved hexose transporter enhances xylose uptake and glucose/xylose co-utilization in Saccharomyces cerevisiae. Scientific Reports, 6(1), 19512. https://doi.org/10.1038/srep19512
-
Stovicek, V., Holkenbrink, C., & Borodina, I. (2017). CRISPR/Cas system for yeast genome engineering: advances and applications. FEMS Yeast Research, 17(5), fox030. https://doi.org/10.1093/femsyr/fox030