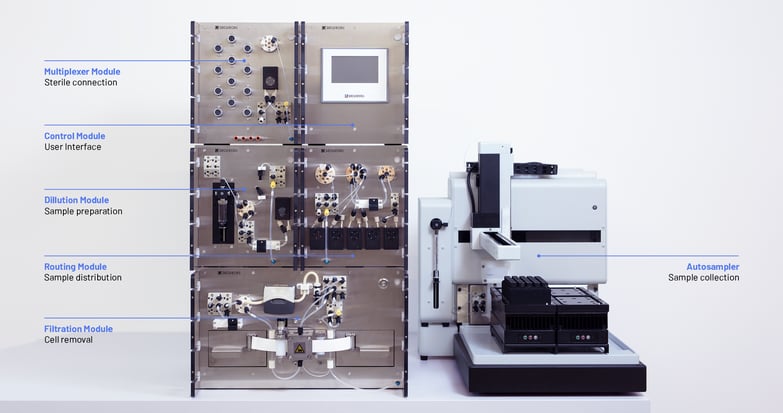
Fully automated sampling with the Numera® system
Biopharmaceutical products are generally heterogeneous in structure due to intra- and extracellular biological, chemical, and physical modifications that take place along the manufacturing process 6. To gain real‐time process information, advanced process analytical technology (PAT) tools are implemented between upstream and downstream unit operations 7. According to Konstantinov and Clooney, a sufficient amount of real-time data around product quality attributes (e.g., aggregation, glycosylation, activity, impurity level) is currently unavailable, and reliable in-line and on-line sensor solutions are unlikely to emerge in the near future 3. Alternative options already implemented today are sterile manual sampling followed by at-line analysis using established analytical methods, which however deliver analytical data with a time delay. With Numera®, sampling and analysis can be completely automated, providing sufficient near real-time information around critical product quality attributes and enabling adaptive process‐wide control.
Numera® can be flexibly configured to allow sampling of up to 16 bioreactors or downstream surge tanks, seamless sample processing, and sample transfer to the Sample Collector for sample storage or 3rd party analyzers (Figure 1). Compared to other sampling systems, Numera® allows for sampling of small sample volumes at a high frequency, reliable sample processing with a precise dilution (error ≤ 2% SD) and unique tape-filtration technology, cooled sample storage in the Sample Collector, and well-established integration with multiple cell and biochemical analyzers and HPLC systems. This results in the complete automation of sample-based monitoring. Additionally, Numera® comes with the powerful process information management software Lucullus® to digitally integrate unit operations and analyzers, collect and centrally store all process data, and allow process-wide monitoring and control.
Seamless integration of sampling-based analytics in continuous bioprocessing
Numera® has been successfully implemented as a reliable sampling system in many research and development laboratories in upstream bioprocessing. In the scope of a continuous bioprocess at a customer installation, Numera® samples for the first time simultaneously along the upstream and downstream processing chain (Figure 2). Revolutionary about the installation is not only the application in both up -and downstream processing with a dual HPLC injection, but also the automated transfer of a deep well plate from the Numera® Sample Collector to a Tecan EVO® platform using an xArm robot. To our knowledge, this is the first report on bringing the Tecan EVO® platform on-line.
The continuous bioprocess is part of an innovation laboratory where the company tests and evaluates cutting-edge bioprocess technologies to reach Bioprocess 4.0 production concepts.
A human recombinant protein important for treating hemophilia is produced by a mammalian expression system in a bioreactor. The outflow consisting of media and cells is transferred to an effluent reservoir and, from there, introduced to the downstream processing workflow (Figure 2, grey line). The downstream unit operations comprising tangential flow filtration, capture liquid chromatography (cLC), and polishing liquid chromatography (pLC) are separated by surge tanks. Surge tanks, which are any type of vessel, tank, or bag placed between unit operations, are often applied to partially or fully decouple unit operations, maintain a smooth continuous operation, and reduce disturbances 8. Additionally, surge tanks are also useful as sampling or insertion points of in-line measurement probes 9.
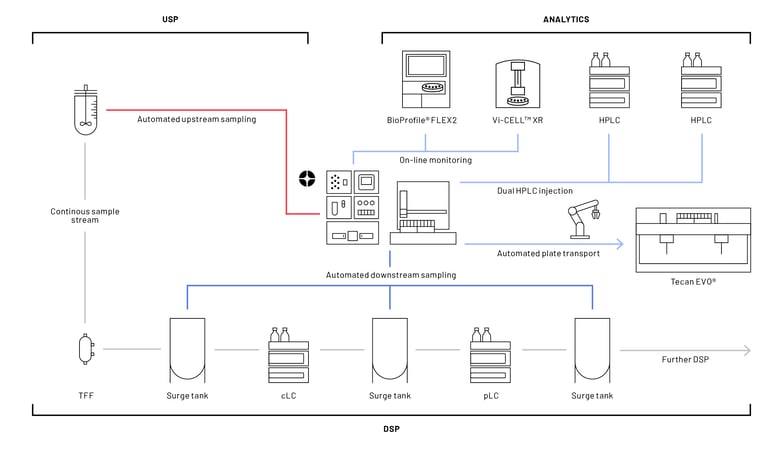
The automated sampling along the continuous bioprocess is realized by multiple fluidic connections between Numera® and the upstream bioreactor systems (Figure 2, red line) and between Numera® and the downstream surge vessels (Figure 2, dark blue line). Numera® sequentially draws samples from either the upstream or downstream vessels, processes the samples, and seamlessly transfers them to the BioProfile® FLEX2 analyzer (NovaBiomedical), the Vi-CELLTM XR analyzer (Beckman Coulter) or stores the samples on the Numera® Sample Collector (Figure 2, light blue line). From the Sample Collector, the samples can be injected into a liquid and/or organic HPLC (Agilent Technologies) and/or transferred within the sample plate via the xArm robot to the Tecan EVO® platform.
Within the presented integrated PAT solution, the manual preparation steps for the sample measurements are automated. These steps include the delivery of the sample to the analyzer, sample naming, and the choice of the analytical method. The real-time analytical results are collected by a supervisory control system and used for the tight monitoring of process variables and product CQA. Additionally, the results are used to adjust the loading duration of the cLC and pLC step in response to decreasing cell-specific productivity to obtain high-capacity utilization without yield loss.
Besides the described integrated analyzers, Numera® has, in another installation, also enabled on-line MD LC-MS charge variant and peptide mapping analysis to monitor multiple post-translational protein modifications, further refining the assessment of critical product quality attributes 10.
Conclusion
Liquid samples are still one of the most important sources of process-relevant data. For example, in upstream processing, almost all information regarding substrates, biomass, and (by)products is based on samples and their analysis. Here, we presented a state-of-the-art automated liquid sampling solution for upstream and downstream processing.
The presented integrated PAT solution provides an answer to basic bioprocess engineering challenges. Based on the full automation, less equipment, consumables, and human resources are needed, errors from manual labor are avoided, high measurement frequency and concomitant high information content are reached, the time delay between sampling and analysis is reduced, and reproducibility is ensured by uniform sample treatment.
With tailored automation and simultaneous digitization, the efficiency of today’s production processes can be significantly increased, forming the foundation of continuous manufacturing, guaranteeing process stability over the product lifecycle, and ultimately resulting in a speed up to the market. The presented platform technology is paving the way for bioprocesses of the future.
References
- Walther, J. et al. The business impact of an integrated continuous biomanufacturing platform for recombinant protein production. J Biotechnol 213, 3–12 (2015).
- Rathore, A. S., Agarwal, H., Sharma, A. K., Pathak, M. & Muthukumar, S. Continuous Processing for Production of Biopharmaceuticals. Prep Biochem Biotechnol 45, 836–849 (2015).
- Konstantinov, K. B. & Cooney, C. L. White Paper on Continuous Bioprocessing. J Pharm Sci 104, 813–820 (2015).
- Nasr, M. M. et al. Regulatory Perspectives on Continuous Pharmaceutical Manufacturing: Moving From Theory to Practice: September 26-27, 2016, International Symposium on the Continuous Manufacturing of Pharmaceuticals. J Pharm Sci 106, 3199–3206 (2017).
- Feidl, F. et al. Process-wide control and automation of an integrated continuous manufacturing platform for antibodies. Biotechnol Bioeng 117, 1367–1380 (2020).
- Walsh, G. Post-Translational Modifications in the Context of Therapeutic Proteins: An Introductory Overview. in Post‐translational Modification of Protein Biopharmaceuticals 1–76 (John Wiley & Sons, Ltd, 2009). doi:https://doi.org/10.1002/9783527626601.ch1.
- Karst, D. J., Steinebach, F. & Morbidelli, M. Continuous integrated manufacturing of therapeutic proteins. Curr Opin Biotechnol 53, 76–84 (2018).
- Thakur, G., Nikita, S., Tiwari, A. & Rathore, A. S. Control of surge tanks for continuous manufacturing of monoclonal antibodies. Biotechnol Bioeng 118, 1913–1931 (2021).
- Patel, B. A. et al. On-Line Ion Exchange Liquid Chromatography as a Process Analytical Technology for Monoclonal Antibody Characterization in Continuous Bioprocessing. Anal Chem 89, 11357–11365 (2017).
- Dahotre, S., Dai, L., Kjenstad, K., Stella, C. & Camperi, J. Real-time monitoring of antibody quality attributes for cell culture production processes in bioreactors via integration of an automated sampling technology with multi-dimensional liquid chromatography mass spectrometry. J Chromatogr A 1672, 463067 (2022).










