Securecell AG/ETH Wyss Zurich: Liver4Life group, September 2021
Keywords: Seraccess®, AGM, blood sampling, in vitro experiment
Introduction
Milestone 5 of the project consists of the evaluation of the automatic blood sampling from an existing peripheral catheter and subsequent extraction of plasma using Securecell's proprietary silicon membrane. This trial was conducted at ETH Zurich, which provided access to a regulated blood circuit. It forms the basis for the first preclinical trial in sheep as of Q2 2022, before the first trials in humans can be conducted.
Automatic blood sampling
3 ml of blood were aspirated for each measurement. A small, undiluted blood sample was then taken for automated glucose measurement. Subsequently, the aspirated blood was returned to the blood system with 10 ml of rinsing solution and ultimately to the patient.
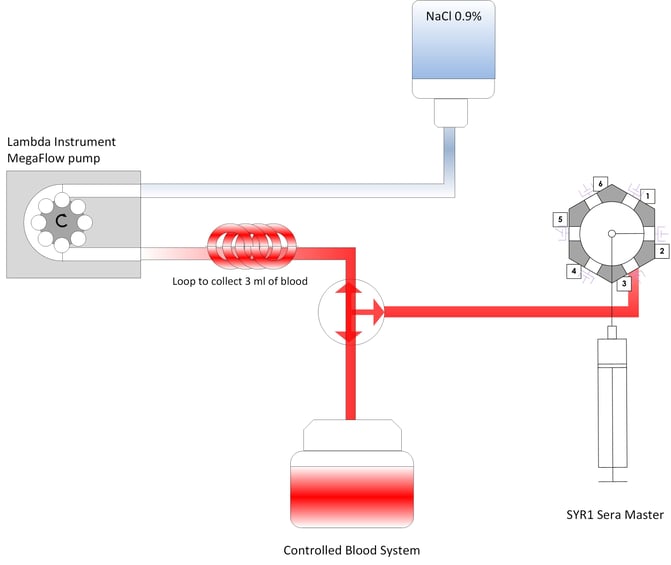
Closed-loop blood sampling systems, as used in ICUs, return un-used blood to the patient. Blood collection and supplying the collected blood sample to the analyzer or forwarding it to the laboratory occurs manually.

Results
The trial was conducted in collaboration with the Liver4Life group (Wyss Zurich) for one week at ETH in September 2021. The automatic sample collection and subsequent plasma extraction were compared to manual sample collection and to plasma extracted from the sample through centrifugation, determining the glucose concentration in both plasma samples using the Cobas C111.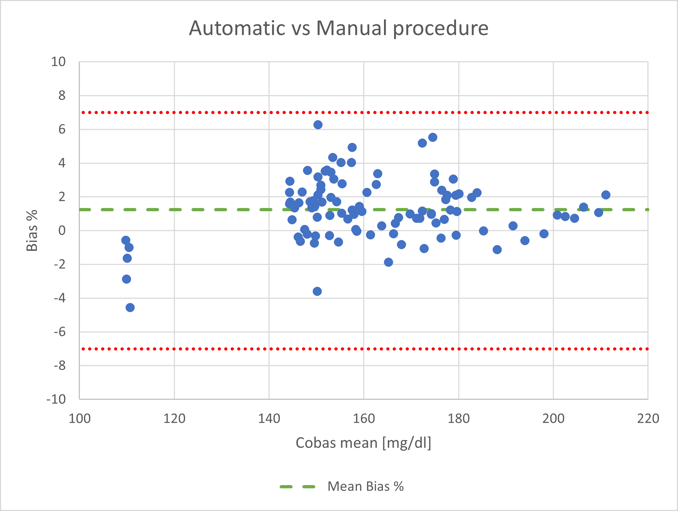
The congruence between automatic and manual blood collection, including sample preparation, was evaluated using a tolerance interval analysis based on the measured glucose concentrations. This analysis shows strong congruence, with 95% of the data points within -2.1 and 4.6%. The bias, the mean deviation of the newly developed automated process compared to the reference method, is 1.3%. The manual method can therefore be replaced by the newly developed automatic blood collection.
|
Milestone 5 was successfully completed. The automatic blood sampling from an existing peripheral catheter, including sample preparation (plasma filtration through the patented silicon membrane), is on par with the classical manual method, the bias is 1.3%. Together with milestone 4 (SeraMaster - intravenous blood glucose measurement versus Cobas C111 - as reference), it forms the basis for the first preclinical trial in sheep as of Q2 2022. |










