Authors: C Bortolussi; Dr. Ch. Ziemba; R. Linkesch; Prof. H. Zulewski; Dr. B. Schär
Presented at the ATTD Advanced Technologies & Treatments for Diabetes Conference, 27–30 April 2022, Barcelona (& online)
Keywords: Automatic glucose monitor, AGM, Artificial Pancreas Device System, APDS
Background and aims
We are developing an innovative blood glucose control technology based on intravenous blood sampling and insulin delivery. Compared to current subcutaneous technologies, the intravenous pathway enables more accurate measurements and removes the time lag between interstitial and blood compartments. A wearable device extracts plasma out of blood, photometrically measures glucose by using the hexokinase reaction and calculates the insulin dosage. Here, we present data of a feasibility study of the new AGM in a clinical environment. Volunteers with diabetes type-1 using a CGM were included, which enabled us to additionally compare the glucose values in venous samples with the sensor results.
Methods
The feasibility study contained eight 6-hour measurement sessions. Blood samples were collected via a PVC every 15-20 minutes and, at the same time, CGM values were recorded. The glucose level in each sample was measured with the new AGM and with Cobas (Roche) for reerence.
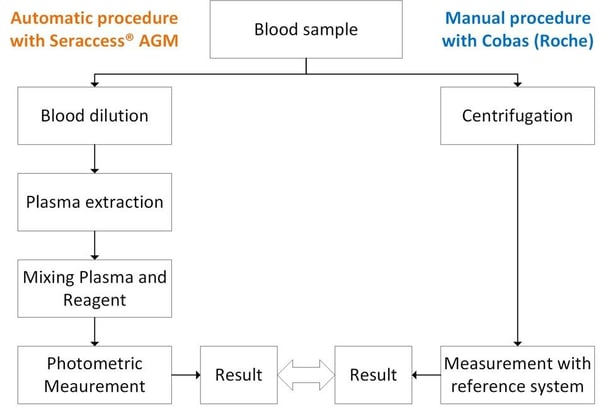
Figure 1: Overview of the procedure: from blood sample until results comparison between Seraccess® AGM and Cobas (Roche)
Results
For method comparison, 196 blood samples in the range 66 mg/dl – 308 mg/dl (3.7 mmol/L – 17.0 mmol/L) were measured using both methods. In the current trial, the correlation was an excellent 0.99, which means that Seraccess® AGM is on a par with the Cobas instrument from Roche, as can be seen in Figure 2.
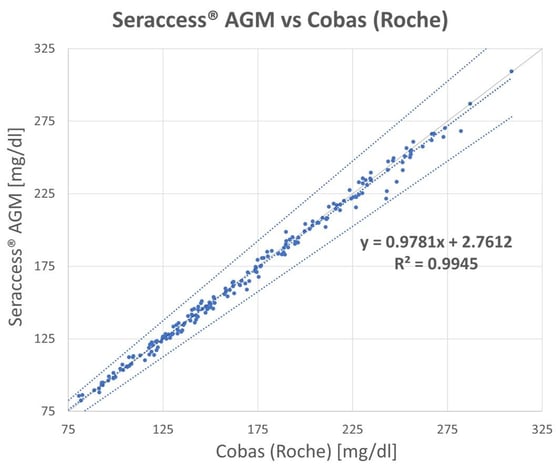
Figure 2: Linear Regression
All test subjects had the readings from Seraccess® AGM and Cobas (Roche) in close alignment. An example can be seen in Figure 3, which shows the blood glucose curve over six hours.
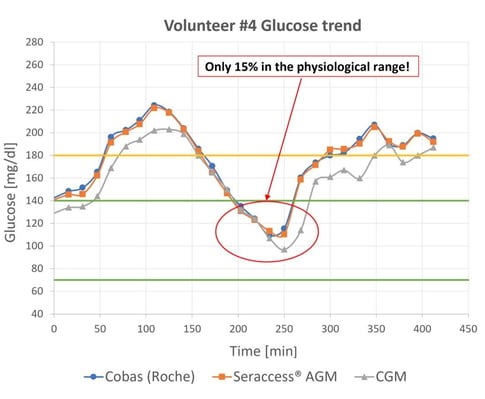
Figure 3: Comparison of Glucose trend with CGM, Cobas (Roche) and Seraccess® AGM
The blood glucose measurement accuracy was evaluated by the mean absolute relative difference (MARD), which was 2.3% (range 1.7% - 3.6%). It was obtained that 100% of the measurements was in the ± 10% error interval. Moreover, 91% of the data points was even within ± 5%. The MARD of the CGM values was 9.6% (range 5% - 24.5%). In order to make insulin dosing decisions based in CGM values with sufficient certainty, the MARD should be below 10%, which was fulfilled on average, but deviated greatly in individual cases.
Conclusions
The results show the good accuracy of the new device compared to the reference method. The Seraccess® technology involves constant, automated, precise glucose measurements that allows to keep blood glucose within the target range. Intravenous sampling eliminates the lag between venous and interstitial compartments, leading to higher performance. The new method is an effective technique to be used in automated insulin delivery systems, combined with closed-loop computation of the insulin quantity.
Abstract published online 25 Apr 2022 Diabetes Technology & Therapeutics Vol. 24, No. S1