Monoclonal antibodies – a strong growth market
In 2021, the global mAb market was valued at USD 186 billion. The North American mAb market, with a market share of 46.2%, continued to expand in 2022. According to a report by Future Market Insights (FMI), the global demand for monoclonal antibodies will increase at a compound annual growth rate (CAGR) of 12% during the forecast period between 2022 and 2032, reaching a total of USD 647.01 billion in 2032. In order to meet the increasing demand for therapeutic recombinant proteins, the development, and improvement of reliable technologies for their efficient production is indispensable.3
Monoclonal antibody expression systems
With the advent of recombinant DNA technology in the 1970s, the first recombinant therapeutic protein Humulin® (human insulin) was expressed from Escherichia coli. The main advantages of recombinant DNA technology are the safe and cost-effective expression of human proteins in host organisms with reduced immunogenicity and the expression of modified protein variants with improved function and specificity, resulting in more reliable products overall. Recombinant protein therapeutics are produced by either prokaryotic or eukaryotic expression systems. Approved cellular host systems for heterologous protein expression include, for example, Escherichia coli, Saccharomyces cerevisiae, Chinese hamster ovarian cells (CHO), baby hamster kidney cells, human embryonic kidney cells, human retinal cells, and mouse myeloma cell lines NS0 and Sp2/0. 2, 6
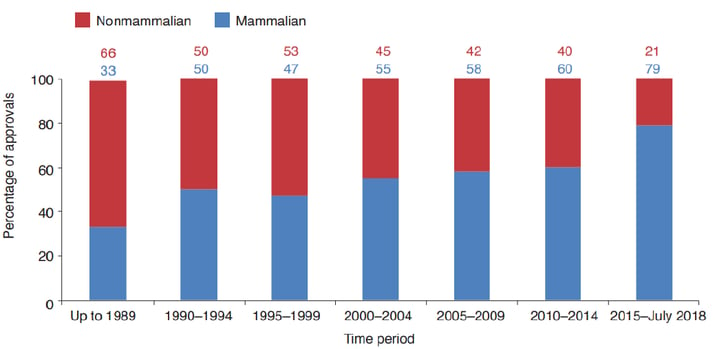
The majority of approved recombinant proteins are produced in mammalian host systems. Post-translational modifications (PTM) may affect the stability, production volumes, pharmacokinetic and pharmacodynamic behavior, safety, and immunogenicity of recombinant protein products. The most common PTM is glycosylation. The ability of the host system to perform correct glycosylation is an important criterion for selection. Mammalian expression systems are often chosen for their ability to synthesize therapeutic proteins with complex glycan structures (Illustration 1).7,8 However, the cultivation of mammalian cells is difficult and expensive compared to non-mammalian cells as they grow comparatively slowly and also produce relatively little protein.
Automation and digitalization to increase production
The main goal of optimizing biopharmaceutical processes is to increase the yield while maintaining a constant quality. Since protein synthesis is still marked by many manual steps, there are many attempts to completely automate production processes. Automation increases the efficiency and yield of the processes, reduces costs, and ensures continuous quality. 9
A success story with a long tradition

Our long-standing customer, whom we have been supporting with our technologies for many years, is active in the development and production of monoclonal antibodies using recombinant CHO cells. The cooperation already began in the 1990s, when our company (at that time named Biospectra AG) developed automation solutions and the first online analytical systems in process development. At that time, the computerization of biotechnology laboratories was still in its infancy and there were few solutions on the market. In 1993, Biospectra began to develop the Lucullus® software for data acquisition and advanced control of bioprocesses. The customer purchased this early Lucullus ® version and used it for data acquisition and process control. The idea behind the software development was to complement the automated sampling systems, the forerunners of today's Numera® system (Illustration 2). Thanks to the intensive cooperation with the customer, the functionality of Lucullus® has been adapted to the needs of the pharmaceutical industry. This cooperation has continued until today and has made a significant contribution to the extensive range of Lucullus® functionalities available today.
Over time, new devices have been integrated and further process steps have been facilitated, such as sample management or shake flask cultivation. Over the last years, the customer has greatly expanded its Lucullus® orchestrated process development department. The complete installation now includes more than 100 bioreactors and equipment. Lucullus® is also used at other company locations and connects researchers from several countries via a uniform platform.
Unified technology - from process development to market production
In 2001, the first GMP pilot plant for the production of monoclonal antibodies on a scale of 1,000 liters was built at the customer's headquarters. Due to the many years of cooperation and the positive experience, the customer decided to implement our technologies. The decision to use Lucullus® as a central data management and process control system was based not only on the comprehensive functionality and proven robustness of the system but also on the simplified and compatible data exchange with the process development departments between the different locations. This was the first time Lucullus® was applied in a GMP environment and marked a milestone in Securecells’s company history.
In 2007, after several expansions and modernizations of the existing cultivation facility, an even larger facility with higher capacity was built in another building at the same site to meet the increasing demand for production batches for clinical trials. The new GMP pilot plant included a cell culture line with 3000 liter reactors. As soon as the plant was completed, a second identical production line was built, doubling the capacity again. In both new plants, our technologies were implemented as well.
Tailor-made automation for a validated environment
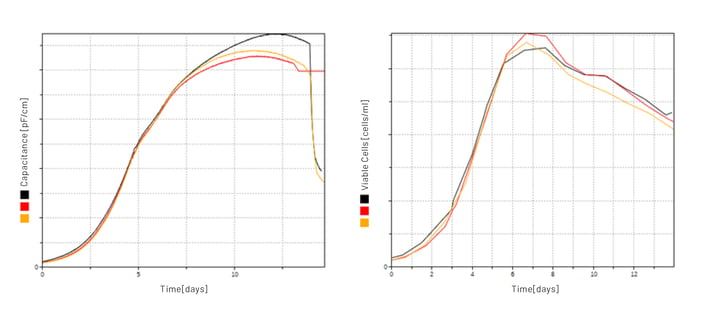
In the course of several modernization steps, the latest technologies were always implemented. A particularly noteworthy innovation of this installation is the use of biomass sensors (Aber Instruments Ltd.), which are used on an industrial scale; one of the first of its kind. Initially, the capacitance measurements were only recorded and compared with the manual cell counts (Illustration 4). After an intensive evaluation of the data and comparison with the measurements from the smaller scales of development, the measurement method was perfected and finally validated over the years together with Aber Instruments. Since then, the signals have been used to control supplementary feeding with Lucullus®.
Owing to these online measurements, further process steps such as temperature or pH changes of the respective production processes were automated and the processes were validated, so that today the entire process can run largely free of manual interactions. This is particularly important in order to be able to operate the plants during the night and over the weekend with little personnel expenditure and to make as much use of them as possible. The programming has been designed in such a way that changes to the recipe or product changes can be implemented without additional validation effort. Our engineers were always involved in the implementation in order to support the customer in the best possible way (Illustration 5).
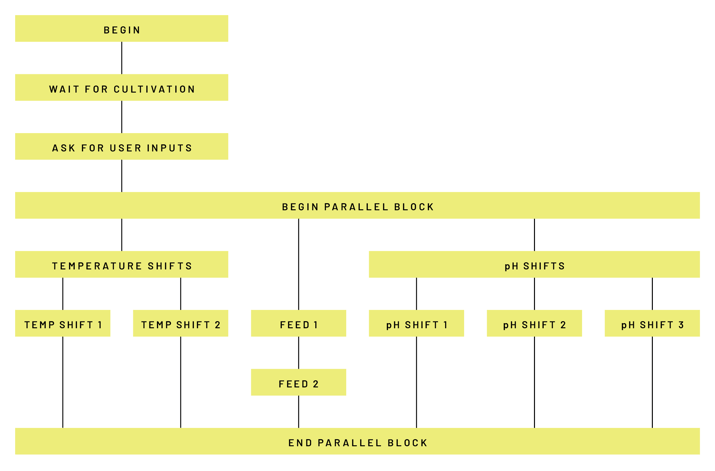 Illustration 5: Lucullus® automated process step chain with the flexible recipe.
Illustration 5: Lucullus® automated process step chain with the flexible recipe.The use of Lucullus® enables GMP-compliant data recording and extended process control in the validated environment and also supports the regulatory documentation of the processes and archiving of all relevant data. These range from measured variables, off-line data, and audit trails, to manual log entries. The entire report creation with comprehensive tables and graphs was just as automated as the process management itself.
With the introduction of the REST-API interface, it became possible to track the processes online via a web interface (Illustration 6) from outside the plant - regardless of location.
 Illustration 6: Process comparison via the web application
Illustration 6: Process comparison via the web applicationFrom pilot plant to commercial production
In view of the steady increase in global sales of the products and limited production capacities, the customer decided in 2018 to convert the two plants for market production. This change was successfully validated, and since then, products for the global market have been manufactured at this site. The transition from piloting to commercial production required comprehensive validation of all processes. We have accompanied the customer in every step of the validation, and Lucullus® has overcome these challenges smoothly.
Soon the launch of the two commercial production plants, their capacity was no longer sufficient to meet market demands and so a further expansion with the aim of doubling the capacity once more is currently being implemented. Thanks to the scalability of Lucullus® and the higher-level functionality, which can be applied to all systems and processes, there is nothing standing in the way of the success of this project.
Conclusion and outlook
Process development, piloting, and production of monoclonal antibodies rely on robust automation and overarching data management encompasses a wide variety of data sources. Lucullus® has proven itself as a comprehensive software solution covering functionality that ranges from data recording, and advanced process control, to tailor-made evaluation, reporting, and convenient web-based monitoring. Lucullus® also meets all requirements of FDA guideline 21 CFR Part 11.10
For more than 30 years now, we have been supporting this customer with our know-how for automation and digitization solutions. Our specialists manage one of the largest Lucullus® installations with a large number of servers and connected devices, such as sensors, controllers, and analyzers, to ensure comprehensive digitization of data and automation of processes.
The close cooperation between the users and our developers is allegorical of the positioning of Securecell AG: We do not simply offer products, but comprehensive solutions that constantly evolve with the needs of the customer.
Learn more about Lucullus

References
- https://www.gkv-90prozent.de/ausgabe/07/kurzmeldungen/07_gamsi-telegramm/07_gamsi-telegramm.html
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat Biotechnol 36, 1136–1145 (2018)
- https://www.prnewswire.com/news-releases/global-monoclonal-antibodies-market-was-valued-at-us-208-32-bn-in-2022-and-is-expected-to-hit-a-revenue-of-us-647-bn-at-cagr-of-12-between-forecast-period-of-2022-32--future-market-insights-inc-301677078.html
- Goeddel, D. V et al. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proceedings of the National Academy of Sciences 76, 106–110 (1979).
- Khan, S. et al. Role of Recombinant DNA Technology to Improve Life. Int J Genomics 2016, (2016).
- Wurm, F. M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22, 1393–1398 (2004).
- Walsh, G. Biopharmaceutical benchmarks 2010. Nat Biotechnol 28, 917–924 (2010).
- Walsh, G. & Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol 24, 1241–1252 (2006).
- https://www.pharma-food.de/organisation/vom-novum-zum-mainstream.html
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/part-11-electronic-records-electronic-signatures-scope-and-application.











