BR1000 Advanced Control Bioreactor System
Using in-line sensors for near-infrared (NIR) and dielectric impedance spectroscopy, the BR1000 bioreactor system (Figure 1) monitors three critical process parameters i.e., glucose, lactate, and VCD in real-time. When employing a custom calibration model, the BR1000 model predictive control (MPC) algorithms are designed to automate the supplemental feeding of glucose to maintain a precise setpoint value concentration. Pertinent to this report, the glucose concentration can also be dynamically controlled at any point during the cell culture timeline.

Figure 1: The Yokogawa BR1000 Advanced Control Bioreactor System.
Lucullus® Process Information Management System
Lucullus® is a powerful software for enterprise-wide bioprocess control and data management. It allows the integration of various bioprocess technology equipment, such as bioreactors, probes, or analytical devices, and further allows process monitoring and control based on the exchanged data. Every step along a typical bioprocess, from design, preparation, execution, and evaluation, is supported by Lucullus®, resulting in a single software solution to manage all data of integrated bioprocesses.
Materials and Methods
Lucullus® Software Information Network
On a standard English Windows-based laboratory personal computer (PC) in the Yokogawa cell culture laboratory, a licensed copy of Lucullus® was installed. The PC was connected via ethernet cable to a network hub that was further connected to each of the three BR1000 bioreactors (LP6, LP7, and PP4), the Roche Cedex® Bio Analyzer, the Beckman Vi-CELL XR™, and a laboratory label printer (Figure 2). Through a bidirectional connection to the bioreactor systems, Lucullus® was able to perform both, the necessary control actions and data collection.
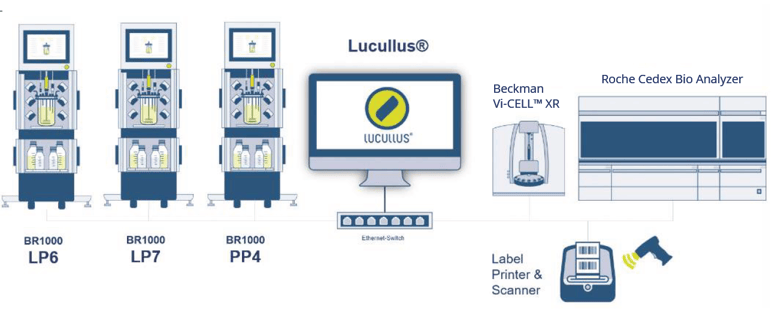
Figure 2: Lucullus ® integrated bioprocess technology setup consisting of three Yokogawa BR1000 bioreactors, a Roche Cedex® Bio Analyzer, a Beckman Vi-CELL™ XR and a label printer.
Prior to daily sample collection from the bioreactors, barcoded labels were printed and attached to culture tubes. The culture tubes then were used for sample transfer from the reactors to the off-line analytical instruments. Based on the barcode, printed on the sample labels, a reference identification between the off-line measurement results and the process and process time was created. This workflow allows the integration of all the off-line data from the off-line analytical instruments in Lucullus®. Thus, the off-line measurements were readily available, with a short time delay, for comparison against and validation of the estimated process values based on the model predictive control algorithms.
The data of the BR1000 reactor measurements and prediction models, Beckman Vi-CELL™ XR measurements, and Roche Cedex® Bio Analyzer measurements were automatically collected in Lucullus®, hence no additional middleware was required for transporting the analytical results.
Cell Culture
A clonal IgG expression CHO cell line (K1SP) was obtained from an external source. Cultivation was performed in FUJIFILM Irvine Scientific CHO cell medium with a starting concentration of 3 g/L of supplemented glucose for all bioreactor runs. Stirred-tank glass vessel designs were employed with a starting volume of 1500 mL and seeded at 150,000 cells/mL with the same seed stock. Standard pH, temperature, gases, and agitation were controlled at previously optimized values and automatically monitored and controlled by the BR1000 bioreactor system. Culture growth was allowed to proceed in parallel for 14 days.
Bioreactor PP4 was the experimental control with a steady-state concentration of glucose maintained at 3 g/L throughout the entire cultivation timeline. The glucose shift in the other two bioreactors, when the cultures reached a VCD of 16 million cells/mL, was automatically controlled and executed based on a custom programmed process control operation by Lucullus®. Bioreactor LP6 was shifted up to 5 g/L and bioreactor LP7 was shifted down to 1 g/L glucose (Figure 3). The employed feed solution was FUJIFILM Irvine Scientific CHO cell medium supplemented with 28 g/L glucose.
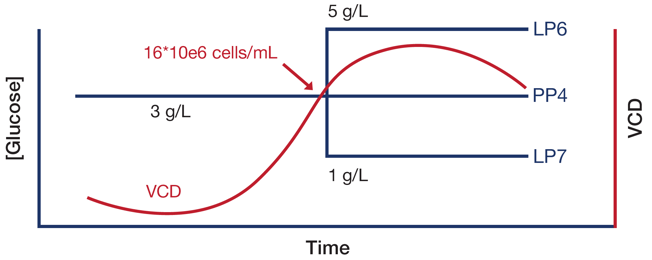
Figure 3: Profile of glucose concentration shift dynamics executed in this study.
Sampling and Off-Line Analysis
Immediately after setup and daily thereafter, a 10 mL sample of the bioreactor culture suspension was extracted for off-line analytical testing. 1 mL sample was mixed with a staining reagent according to the protocol and analyzed with the Beckman Vi-CELL™ XR to determine total cell count and VCD. The other portion was centrifuged to pellet cells and a portion of the supernatant was analyzed with the Roche Cedex® Bio Analyzer for glucose, lactate, and IgG concentration. The remaining sample was placed in storage at -20 °C (-4 °F) for possible future characterization of the secreted antibody product.
Results
Cell Growth Dynamics
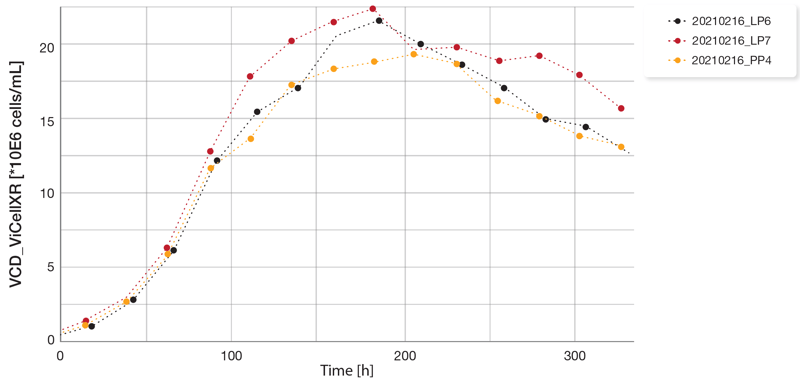
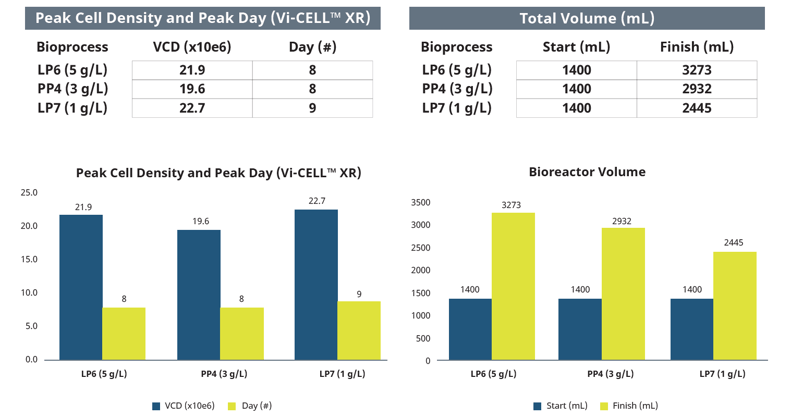
Across the three bioreactors, cell growth was comparable with a peak VCD of around 20 million cells/mL on about the eight day of cultivation (Figure 4). Due to the different setpoints of glucose concentration, the feed volume varied, resulting in significant differences in the final culture volume. The final culture volume of the LP6 bioreactor with a glucose setpoint of 5 g/L was over 2.3 times higher than the starting volume. The final culture volume of the other two bioreactors was progressively lower consistent with the glucose concentration setpoint (Figure 5).
Comparison of Estimated and Off-Line Parameters
Across the three bioreactors, the glucose and lactate concentration, and the VCD were measured with off-line analysis (Roche Cedex® Bio Analyzer, Beckman Vi-CELL™ XR) and estimated based on the BR1000 integrated MPC (Figure 6).
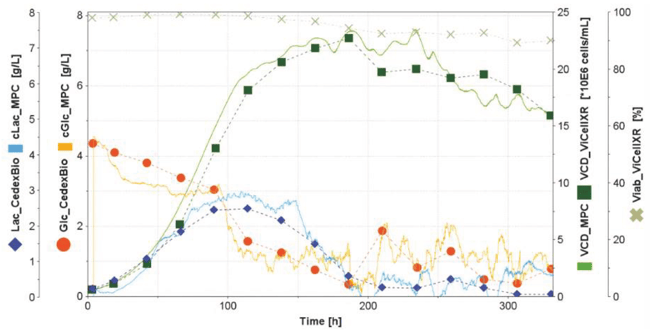
Figure 6: An overlay of the at-line reference measurements and corresponding estimations with the MPC algorithm shows agreement between the signals (exemplarily shown are data from the LP7 bioreactor).
Under ideal circumstances, the off-line measured and BR1000 MPC-estimated data sets are in close alignment. Generally, the off-line data is considered the true “reference data,” as the assays have been used extensively for years throughout the industry and display high reliability. Control of the bioreactor, however, is managed using the soft-sensor MPC data derived from the BR1000 in-line sensor systems. To determine the degree of similarity between the MPC data and the reference data, the values were compared using the root mean squared error of prediction (RMSEP) statistic (Figure 7). To calculate RMSEP, a custom script (LCALC script) was programmed in Lucullus® according to the formula in Figure 7.
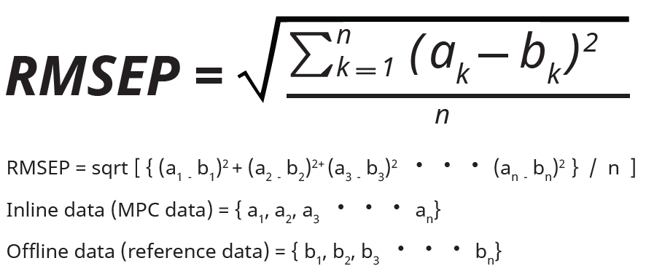
The script provided a continuous update of the underlying statistical calculation and a final single value for each of the soft-sensor parameters for all bioreactors (Figure 8). A perfectly aligned comparison between off-line and estimated values would have a theoretical value of 0.000. The RMSEP values for glucose and lactate ranged from 0.320, representing the closest observed alignment (LP7, Lactate), to 0.800, representing the poorest aligned case (PP4, Lactate). RMSEP values for VCD over the whole process ranged from 1.534 (LP7) to 5.342 (PP4). The alignment of the off-line and estimated VCD values was more accurate in the exponential growth phase than in the stationary and early dead phase. Overall, off-line measured and MPC estimated glucose, lactate and VCD values are comparable (Figure 6 and 8).
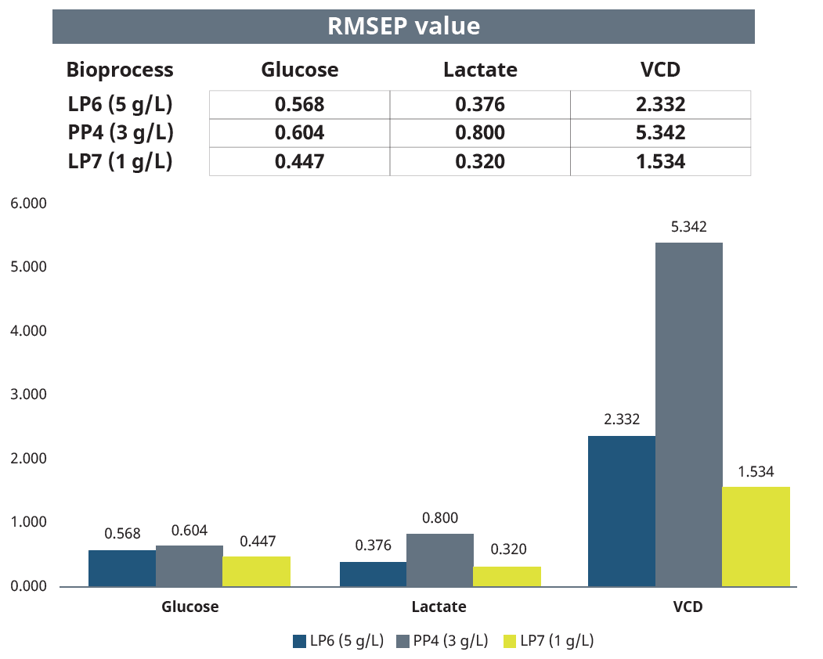 Figure 8: RMSEP values calculated by Lucullus® for glucose, lactate, and viable cell density reported and compared.
Figure 8: RMSEP values calculated by Lucullus® for glucose, lactate, and viable cell density reported and compared.
Antibody Yields
Along with the other critical culture parameters, the concentration of IgG antibody produced was assessed daily using the Roche Cedex® Bio Analyzer. The IgG antibody concentrations varied only slightly between the three cultures (Figure 9). However, the final culture volumes varied significantly, and as a consequence, the total yields differ, with the lowest total yield for the LP7 (1 mg/L glucose) and the highest total yield for the LP6 (5 mg/L glucose) bioreactor culture.
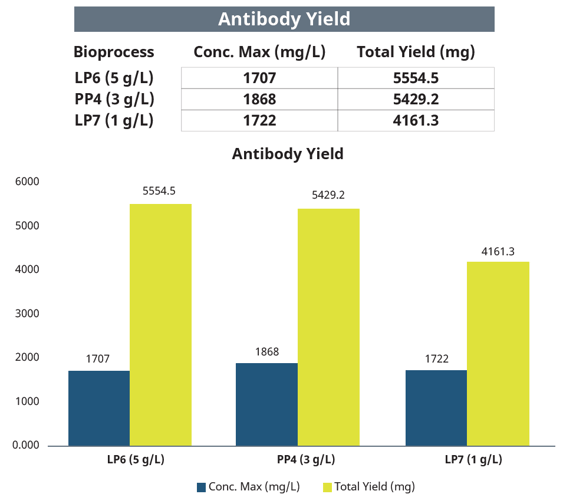 Figure 9: Antibody concentrations were determined by the Roche Cedex Bio Analyzer with an IgG-specific immunoreagent assay. Results are shown for the final day (Day 14).
Figure 9: Antibody concentrations were determined by the Roche Cedex Bio Analyzer with an IgG-specific immunoreagent assay. Results are shown for the final day (Day 14).
Discussion
The conducted experiment was programmed to trigger a dynamic glucose concentration shift at a VCD of 16 million cells/mL in two of the cultures (LP6, LP7). The monitoring and execution of the glucose shifts at the precise VCD were carried out by a Lucullus® embedded process control operation. Lucullus® ensured that the concentration shifts were synchronized properly and initiated independent of human involvement, thereby eliminating any possible user error.
Each of the cultures appeared to reach very similar peak VCD. Although the RMSEP values for VCD demonstrated a higher reported overall number and greater variability than the RMSEP values for glucose and lactate, a direct comparison is inappropriate due to different measurement scales and units. Furthermore, the RMSEP values calculated before reaching peak VCD showed little variation.
The RMSEP values for glucose and lactate revealed that the NIR sensing and MPC with the BR1000 bioreactors performed well enough to provide good estimates for glucose and lactate concentrations consistent with the actual off-line determined measurements. The RMSEP values ranged from 0.320, representing the closest observed alignment (LP7, Lactate), to 0.800, representing the poorest aligned case (PP4, Lactate).
Interestingly, the LP7 (1 g/L glucose) bioreactor displayed the highest VCD but the lowest total yield of monoclonal antibodies. The other two cultures, PP4 (3 g/L glucose) and LP7 (5 g/L glucose) both achieved about 20% greater total yields. It is also noteworthy that the feeding regimen employed was using a 28% glucose solution delivered in a complete (sterile) CHO cell media preparation. As such, cultures maintained at a higher glucose concentration were provided with greater feed volumes of glucose and all fresh nutrient components, ending with the greatest final total volume (LP6, 5 g/L glucose, 3273 mL).
Based primarily on the RMSEP values of glucose and peak VCD, it can be concluded that the bioreactor performance of the three BR1000 units was consistently good overall, and variation in the observed final yield is likely not an artifact of glucose feeding or bioreactor-induced cell growth discrepancies.
Conclusion
Dual Scientific Purposes
This experiment had the dual purpose of being both scientifically objective and subjective. The objective purpose was to study and optimize glucose requirements for maximal monoclonal antibody productivity. The subjective purpose was to explore the potential benefits provided by combining the use of multiple BR1000 bioreactor units with the process information management capabilities of Lucullus®.
Regarding the glucose optimization, the highest IgG yields were found for cultivations at 3 g/L to 5 g/L glucose after peak VCD in stirred tank fed-batch culture using the BR1000 bioreactor systems. The possibility of any qualitative differences between the monoclonal antibody produced at these concentrations was not explored, especially with respect to any antibody CQAs. Typical CQA might include glycosylation profiles, charge variant profiles, or other biochemical parameters. From the daily collected samples, aliquots were measured (i.e., monoclonal antibody titer, VCD, glucose, lactate), and some material was frozen for possible future analysis (TBD). Until such time that the samples can be further characterized, it is reasonable to conclude that a consistent concentration of 3 g/L glucose is optimal and more economical for the cultivation of the K1SP CHO cell line to maximize monoclonal antibody production.
Regarding the more subjective assessment of the Lucullus® empowered BR1000 bioreactor systems, the following can be concluded:
-
Consolidated Operations: The ability to control multiple laboratory instruments from one human-machine interface is a key design feature of Lucullus®. For that purpose, it worked extremely well, allowing users to start, monitor, and control three bioreactors simultaneously with all process parameters available on one screen.
-
Sample Planning and Tracking: Barcode labels were designed and then printed on a typical office printer. After sampling the bioreactor, the sample tube was labeled with the respective barcode for reference identification between the off-line measurement results and the process and process time. Off-line measurement results were automatically imported in Lucullus® and assigned to the corresponding process and process time.
-
Customized Control Automation: Initiating the glucose shift in the two bioreactors at a designated VCD was executed automatically and flawlessly during off-hours based on a Lucullus® customer-specific process control operation with no human monitoring.
-
Calculation Scripts for Process Insights: The programming of the Lucullus® LCALC script for the RMSEP calculation was intuitive and the data was an important control for comparing multiple bioreactor units. Only this one LCALC script was employed, although there are ideas for several useful others.
-
Real-Time Convenient Data Display Graphics: Lucullus® provided great flexibility to sort, cut, and display data in many different graphical formats with data from a single bioreactor or multiple bioreactors in one graph and also process comparison of currently running processes to historic process data.
-
Remote Monitoring and Control: Remote programming and control of the bioreactor units from Securecell engineers in Switzerland at Yokogawa in Japan during this experiment demonstrated the remote access and control possibilities.
-
Data Backup and Integrity: The bioreactor data was routinely stored in each of the BR1000 bioreactor units, while also automatically saved in the Lucullus® database for complete data integrity across all historic and currently running processes.
The BR1000 Advanced Control Bioreactor System from Yokogawa Life Science exhibited reliable process performance and MPC-based estimations of process values across the three bioreactor systems. The Lucullus® Process Information Management System from Securecell AG provided laboratory instrument data management and control capabilities, including flexible programming, which created benefits for efficient execution of process development experiments plus data integrity and storage.
In conclusion, in this application note, Yokogawa and Securecell AG presented a state-of-the-art platform for efficient biopharmaceutical process development and optimization with minimized user interactions, no process disturbance, complete process control, and full data integrity.










