Introduction
In the European Union and the United States, monoclonal antibodies represent the most often approved biopharmaceutical product, followed by hormones, enzymes, vaccines, nucleic acid-based products, and modified cell-based products. Most biopharmaceutical products are produced by cellular expression systems in complex bioprocesses [1]. The maintenance of cell integrity during all stages of a bioprocess is essential for optimal cell growth, culture longevity, high product titers, and product quality. Various bioprocess variables and operating conditions influence cell integrity parameters as e.g., basal and supplemental media composition, aeration system parameters, agitation parameters, baffle parameters, the heating system, and the feeding strategy. To achieve optimal bioprocess performance, frequent monitoring of cell integrity parameters, specifically cell viability and viable cell density (VCD), is crucial [2]. VCD is one of the most important key performance indicators of bioprocesses and VCD measurements are also commonly used to control the timing of specific process events such as inoculation, induction, feeding, or culture harvest to ensure optimal culture productivity. Despite considerable progress in on-line and software-supported monitoring technologies, it is most often not possible to fully circumvent liquid (i.e., cell suspension) samples as a reliable source for information on culture viability. However, the manual sampling of a culture followed by off-line VCD measurements is a tedious, labor-intensive, and time-consuming procedure [3]. The next paragraph presents Securecell’s innovative bioprocess automation tools Lucullus® and Numera® in combination with the Cedex® HiRes Analyzer (Roche Diagnostics GmbH) as an optimal consorted solution for automated on-line monitoring and control of critical cell integrity parameters.
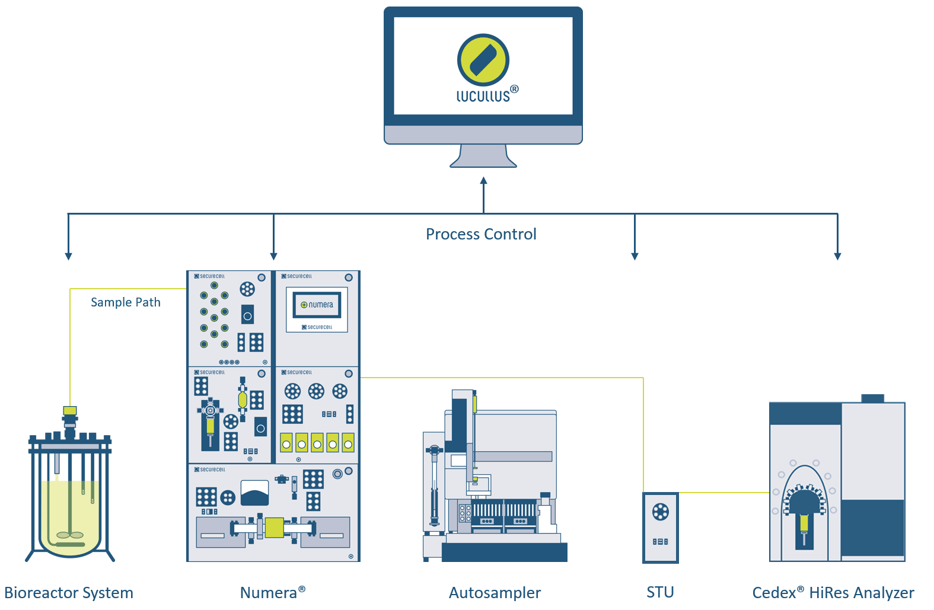 Figure 1: System overview. The used infrastructure has two levels: i) the hardware level including the bioreactor system (with scales, pumps, in-line probes), the automated sampling system Numera® (composed of Multiplexer Module, Control Module, Dilution Module, Routing Module, and Filtration Module from top left to bottom right) with an autosampler, a sample transfer unit, the Cedex® HiRes Analyzer, and an HPLC system (not shown). ii) the overarching software Lucullus® for the planning, preparation, execution, and evaluation of all processes. No additional middleware is required. The sample path for automated sampling and on-line analysis is emphasized with a green line.
Figure 1: System overview. The used infrastructure has two levels: i) the hardware level including the bioreactor system (with scales, pumps, in-line probes), the automated sampling system Numera® (composed of Multiplexer Module, Control Module, Dilution Module, Routing Module, and Filtration Module from top left to bottom right) with an autosampler, a sample transfer unit, the Cedex® HiRes Analyzer, and an HPLC system (not shown). ii) the overarching software Lucullus® for the planning, preparation, execution, and evaluation of all processes. No additional middleware is required. The sample path for automated sampling and on-line analysis is emphasized with a green line.
Integration of the Cedex® HiRes Analyzer into the Numera® PAT system
The modular automated sampling system Numera® can be flexibly configured with the following modules: a Control Module, 1-4 Multiplexer Module(s), a Routing Module, a Dilution Module, a Filtration Module, and an autosampler (Figure 1). Numera® allows for sampling of up to 16 bioreactors in parallel, seamless sample processing, and sample transfer to the autosampler or 3rd party analyzers. The autosampler is used for sample collection and storage at 4 °C. The Cedex® HiRes Analyzer is an image-based cell culture analyzer providing information about cell density, cell viability, cell aggregation, and morphological parameters such as cell diameter and cell compactness. The analyzer assesses these parameters using the trypan blue exclusion method in combination with a high-resolution image scanner. The trypan blue exclusion method is based on the principle that viable cells possess intact cell membranes and exclude the trypan blue dye whereas cells undergoing apoptosis or necrosis lose membrane integrity and take up the dye [4].
The sample transfer from the Numera® to the Cedex® HiRes Analyzer is realized by connecting the Numera® Routing Module and the Cedex® HiRes Analyzer with a sample transfer unit (STU) specially engineered by Securecell for this integration. The STU holds a 6-way loop valve and a liquid sensor (Figure 2). The sample is transferred from the Numera® Routing Module pump via the 6-way loop valve until the liquid sensor. Upon detection of the sample at the sensor, valve rotation connects the filled 300 µL sample loop to the Cedex® HiRes syringe. The modularity of the system and automated liquid detection allows for some flexibility with regard to the spatial configuration and sample line lengths. As soon as the measurement on Cedex® HiRes Analyzer is finished, the results are automatically transferred to Lucullus®. The sample analysis method of the on-line and off-line Cedex® HiRes samples is similar i.e., cellular features are common and results are comparable. An identical sample transfer mechanism with minor changes on the STU is used with cell analyzers from other suppliers.
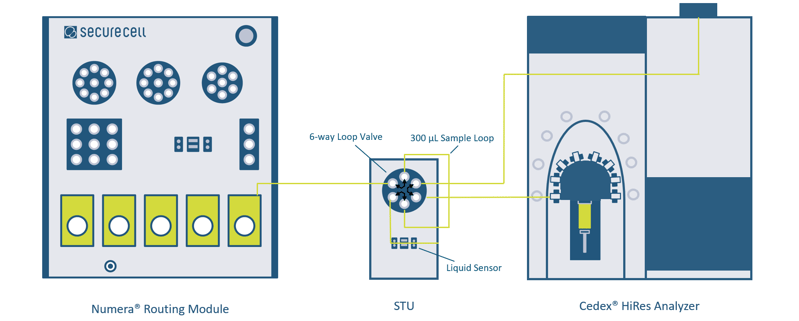 Figure 2: System detail. Schematic of the fluidic connection with a sample transfer unit between the Numera® Routing Module and the Cedex® HiRes Analyzer. For the integration of a Cedex® HiRes Analyzer at least a Numera® Control Module, a Multiplexer Module, a Routing Module, and a sample transfer unit are required. The Dilution Module is optional. The sample path is emphasized with a green line.
Figure 2: System detail. Schematic of the fluidic connection with a sample transfer unit between the Numera® Routing Module and the Cedex® HiRes Analyzer. For the integration of a Cedex® HiRes Analyzer at least a Numera® Control Module, a Multiplexer Module, a Routing Module, and a sample transfer unit are required. The Dilution Module is optional. The sample path is emphasized with a green line.
In-line dielectric spectroscopy
Permittivity is a material property that is measured using dielectric spectroscopy and takes effect when a material interacts with an electric field. The charge carriers of the material orient themselves to the vector of the electric field and create a polarization field that opposes and weakens the external field. The larger the tendency for charge distortion (also called electric polarization) of the material, the larger the value of the permittivity. Cell permittivity measurements are often used for the estimation of overall VCD due to the fact that viable cells polarize whereas dead cells have damaged membranes and do not polarize anymore. However, larger cells polarize more i.e., larger cells make up a proportionally larger part of the signal than the same number of smaller cells. Therefore, when an electric field is applied to cell cultures, the permittivity measurement should be proportional to the total VCD but fundamentally represents a measurement of viable cell biovolume [3,5].
Material and Methods
Set-up automated sampling
All experiments described were performed in a bioprocessing laboratory with a fully integrated IoT infrastructure (i2BPLab) at Zurich University of Applied Sciences (ZHAW) Wädenswil. In the presented application, an unprocessed sample fragment was transferred from the Numera® Routing Module to the STU and subsequently to the Cedex® HiRes Analyzer for on-line analysis (system parameters: focus offset 25 µm, sedimentation duration 60 s, CMinSize 7, CMaxSize 70, AggrMinSize 12). All devices including the reactor system, the Numera®, the Cedex® HiRes Analyzer, and an HPLC system were connected to Lucullus®. In Lucullus®, the samples were planned and triggered during the process execution phase, and all data were recorded, including data from manually drawn and analyzed samples. Automated sampling and analysis with the Cedex® HiRes Analyzer were performed about every 3-6 h and manual sampling approximately every 24 h. The total process duration was 169 h including a 20 h preparation phase for a sterility check and media conditioning phase.
Cultivation condition
Chinese hamster ovary (CHO) cell fed-batch cultivations were performed in a 2.5 L Labfors 5 bioreactor (Infors HT) equipped with an Incyte sensor probe (Hamilton AG) for in-line cell permittivity measurements and a dip tube attached through PG13.5 port. The dip tube was connected to the Numera® Multiplexer Module for sample drawing. The reactor was supplied with air (0.05 Ln/min), O2 to control dissolved O2 at 40%, and CO2. The temperature and stirrer setpoints were set at 37 °C and 100 rpm, respectively. The pH was controlled at 7.2 by the CO2 flow and base supplementation.
Results and Discussion
The execution of one complete automated on-line analytical run on the Numera® Cedex® HiRes system takes less than 15 min, including Numera® sample drawing, direct sample transfer, analysis by the Cedex® HiRes Analyzer, and cleaning of the sampling lines. Figure 3 exemplarily demonstrates the versatility of the system by providing frequent and reliable measurements of cell viability with the Cedex® HiRes Analyzer and lactate with an HPLC system, as well as the flexibility of Numera® to integrate multiple analyzers simultaneously.
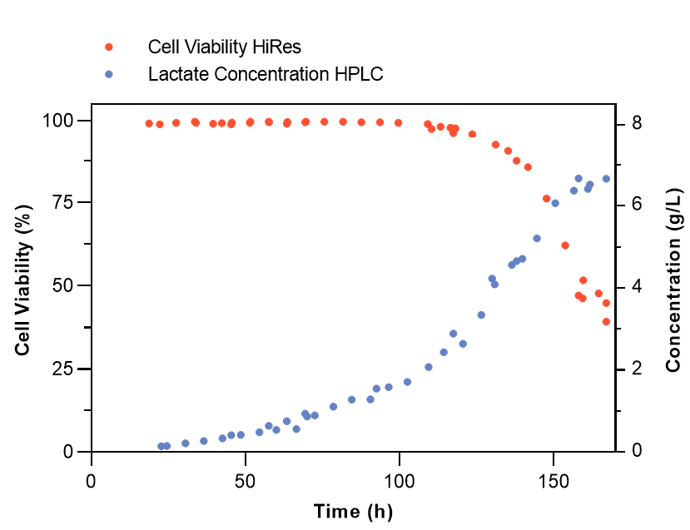 Figure 3: Automated on-line monitoring of cell viability (orange dots) and lactate concentration (blue dots) during a CHO fed-batch process.
Figure 3: Automated on-line monitoring of cell viability (orange dots) and lactate concentration (blue dots) during a CHO fed-batch process.
The performance of the Numera® Cedex® HiRes system was validated by comparison to an in-line and off-line measurement approach. Methods to measure in-line cell integrity parameters include for example 3D digital holographic microscopy or in-situ spectroscopy such as Raman, fluorescence, and dielectric spectroscopy. Here, the in-line permittivity measurements were taken with an Incyte dielectric spectroscopy probe and the off-line measurements with the Cedex® HiRes Analyzer after manual sampling of the reactor system. Permittivity measurement values (pF/cm) correlate with the VCD (cells/mL) and would require a conversion factor to translate the permittivity to an absolute VCD value.
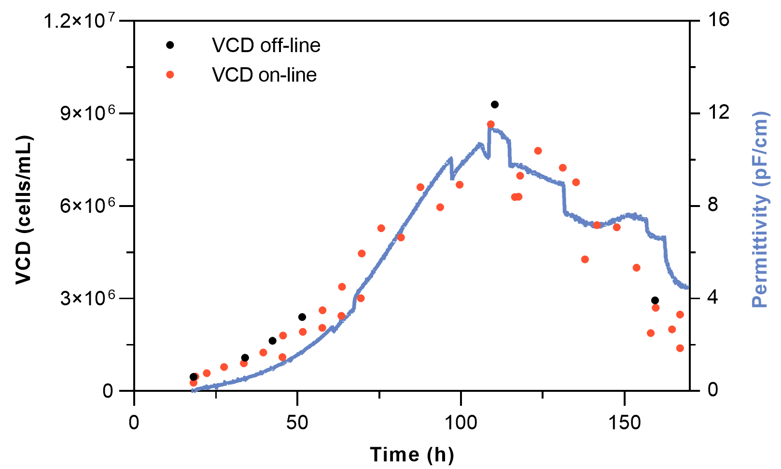
All three measurement techniques (in-line, on-line, and off-line) are reliable approaches to monitoring the VCD (Figure 4). The clear advantage of the on-line over the off-line VCD monitoring method is the possibility of a high measurement frequency while requiring fewer operators and manual actions. The fully automated approach also reduces the risks of contamination, manual errors, operator-to-operator variations, and system parameter fluctuations that potentially result in cellular stress which might negatively influence product titer and quality.
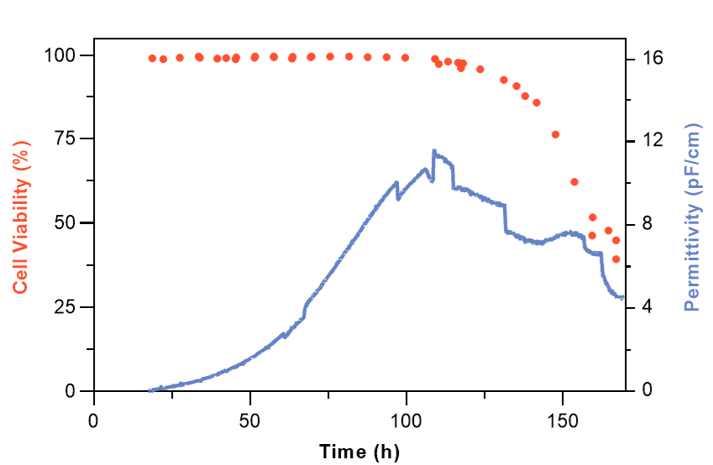
Both, the in-line and on-line measurement approaches allow for VCD monitoring at a high frequency. The minor fluctuations of the permittivity signal at 80-120 h process time coincide with antifoam and glucose addition. At 150 h process time divergence between the in-line and on-line VCD measurement signal is observed. At this stage, the culture is in the death phase with declining overall cell viability (Figure 5). According to Opel et al., the in-line permittivity signal during the death phase of a mammalian CHO culture does not correlate well with the VCD since the cell diameter of apoptotic cells is increasing. As permittivity captures changes in cell biovolume, dielectric spectroscopy measurements during the death phase are less reliable 6,7. Further, it is not possible to discriminate from the permittivity signal alone whether a signal decrease is due to viability loss or a reduction in the cell number.
For the on-line Cedex® HiRes determined VCD, minor variations between subsequent measurements are observed (Figure 4). The variation can be explained by: 1. the inherent standard error of the Cedex® HiRes Analyzer, 2. incomplete sample loop filling of the STU i.e., when not exactly 300 µL sample are transferred to the analyzer, 3. sample line gradients also known as chromatographic effects.
Conclusion and Outlook
The Cedex® HiRes Analyzer was successfully integrated with Securecell’s automated sampling system Numera® and the performance was tested during a mammalian cell cultivation process. The on-line VCD measurement with the Numera® Cedex® HiRes system provides a robust solution to monitor multiple cell integrity parameters in high frequency and real-time without the need for manual interaction. Together with Lucullus®, the presented setup is a state-of-the-art system for automated monitoring and control of critical cell integrity parameters using the well-established trypan blue exclusion method.
Work is in progress to confirm these initial results and to further improve the system in terms of precision i.e., the sampling time of Numera® should be increased to ensure complete sample loop filling of the STU and shorter connection tubing between the Numera® and the STU should be used to reduce sample line gradients.
Key Results:
-
On-line availability of reference analytics (Cedex® HiRes Analyzer)
-
Full automation of the well-established trypan blue exclusion method
-
The online VCD/viability measurements correlate to the offline/manual measurements
-
Centralized and advanced bioprocess monitoring and control enabled by Lucullus®
-
Combination of Numera® and Lucullus® as integrated PAT solutions
References
-
Walsh, G. Biopharmaceutical benchmarks 2018. Nature Biotechnology 36, 1136–1145 (2018).
-
Grilo, A. L. & Mantalaris, A. Apoptosis: A mammalian cell bioprocessing perspective. Biotechnology Advances 37, 459–475 (2019).
-
Strober, W. Trypan Blue Exclusion Test of Cell Viability. Current Protocols in Immunology 21, A.3B.1-A.3B.2 (1997).
-
Opel, C. F., Li, J. & Amanullah, A. Quantitative modeling of viable cell density, cell size, intracellular conductivity, and membrane capacitance in batch and fed-batch CHO processes using dielectric spectroscopy. Biotechnology Progress 26, 1187–1199 (2010).
-
Metze, S., Ruhl, S., Greller, G., Grimm, C. & Scholz, J. Monitoring online biomass with a capacitance sensor during scale-up of industrially relevant CHO cell culture fed-batch processes in single-use bioreactors. Bioprocess and Biosystems Engineering 43, 193–205 (2020).