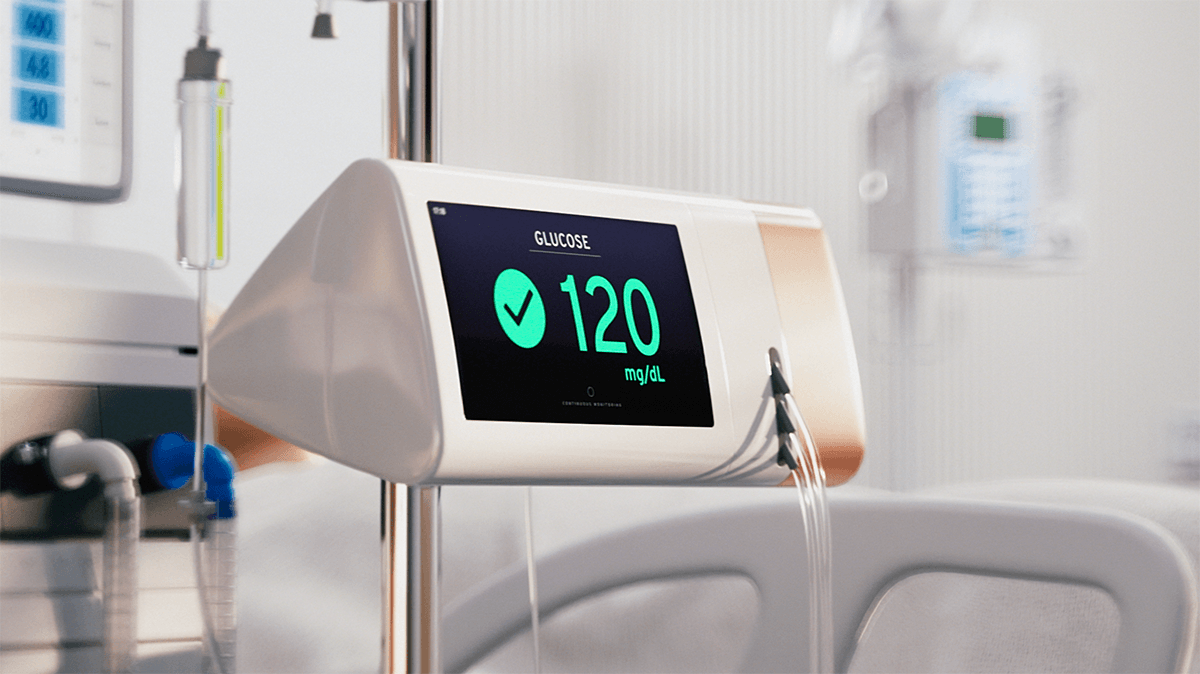
Seraccess® ONE / NEXTRA APDS (Artificial Pancreas Device Systems)
In addition to Seraccess® ICU devices, Securecell is developing two APDS devices that will provide the highest quality automated care to diabetics living with moderate to severe glycemic dysregulation unprecedented freedom to live in control.
Why Seraccess® ONE?
Seraccess® ONE is a wearable device which operates nearly in real time and in direct communication with the patient's bloodstream. The nearly delay-free, highly accurate and precise blood-glucose measurement allows to administer the exact amount of insulin needed - via a commercially available standard catheter - directly into the bloodstream to manage blood glucose within the target range. An increase in time in range translates directly into a reduction of short- as well as long-term complications. Seraccess® ONE is a fast acting closed-loop control system which can revolutionize therapy in this patient group.
Seraccess® ONE - A Breakthrough Wearable Artificial Pancreas for Diabetics with Severe Glycemic Dysregulation
Seraccess® ONE is a fully automated, semi-miniature wearable closed-loop APDS for highly accurate and precise blood-glucose monitoring and control for the diabetes patients who need it most. Seraccess® ONE operates with a commercially available standard catheter.
Brittle diabetes describes a group of diabetes patients suffering from life-changing disruptions due to severe glycemic dysregulation, indicating a condition of instability of glycemic control requiring frequent hospitalizations and causing impaired quality of life and enormous economic cost. These patients are at risk for many unfortunate consequences: poor glycemic control with its complications, more emergency room and hospital admissions, strains on relationships, and greater healthcare utilization and diabetes distress. Current standard of care cannot significantly help these patients, they spend very little time within the target blood glucose range.1 Brittle diabetes is characterized by severe instability of blood glucose levels with frequent and unpredictable episodes of hypoglycemia often requiring hospitalization. Hypoglycemia unawareness is one of the hallmarks of brittle T1D. Hypoglycemia unawareness is especially dangerous because the hypoglycemic individual will not know to take corrective action to prevent further deterioration. If left untreated, hypoglycemia may become severe, resulting in confusion, disorientation, loss of consciousness, or, in extreme cases of prolonged hypoglycemia, permanent brain damage or death.2 Equally dangerous are pronounced and prolonged hyperglycemias, which can lead to diabetic ketoacidosis (DKA) and associated frequent hospitalizations.
Secondary complications, including neuropathy, cardiovascular disease, and retinopathy can be especially common in brittle T1D and there is a significant excess mortality in these patients despite intensive insulin therapy.3 A twenty-year outcome study showed a shockingly 50% mortality rate in a group of women with a mean age of 42 years. In a normal non-brittle type 1 diabetes population, the expected death rate over such a period would be about 5%.4,5
Fully miniaturized and automated, wearable closed-loop APDS for highly accurate and precise blood-glucose monitoring and control for the diabetes patients who need it most and beyond. Seraccess® NEXTRA operates with a proprietary transdermal port (SeraPort), the sophisticated architecture of which makes the sampling of minuscule amounts of blood possible in the first place. Only 20µl to 30µl of blood are needed to perform a highly accurate and precise blood glucose measurement. Seraccess® NEXTRA will be the first and only fully miniaturized and automated, wearable technology for the treatment of diabetes which communicates and interacts directly with the blood – and therefore the only solution which can meet the requirements for a true artificial pancreas. Not only for patients suffering from severe glycemic dysregulation, but also beyond into the broader indication of moderate glycemic dysregulation.
Brittle diabetes describes a group of diabetes patients suffering from life-changing disruptions due to severe glycemic dysregulation, indicating a condition of instability of glycemic control requiring frequent hospitalizations and causing impaired quality of life and enormous economic cost. These patients are at risk for many unfortunate consequences: poor glycemic control with its complications, more emergency room and hospital admissions, strains on relationships, and greater healthcare utilization and diabetes distress. Current standard of care cannot significantly help these patients, they spend very little time within the target blood glucose range.1 Brittle diabetes is characterized by severe instability of blood glucose levels with frequent and unpredictable episodes of hypoglycemia often requiring hospitalization. Hypoglycemia unawareness is one of the hallmarks of brittle T1D. Hypoglycemia unawareness is especially dangerous because the hypoglycemic individual will not know to take corrective action to prevent further deterioration. If left untreated, hypoglycemia may become severe, resulting in confusion, disorientation, loss of consciousness, or, in extreme cases of prolonged hypoglycemia, permanent brain damage or death.2 Equally dangerous are pronounced and prolonged hyperglycemias, which can lead to diabetic ketoacidosis (DKA) and associated frequent hospitalizations. Secondary complications, including neuropathy, cardiovascular disease, and retinopathy can be especially common in brittle T1D and there is a significant excess mortality in these patients despite intensive insulin therapy.3 A twenty-year outcome study showed a shockingly 50% mortality rate in a group of women with a mean age of 42 years. In a normal non-brittle type 1 diabetes population, the expected death rate over such a period would be about 5%.4,5
SERACCESS NEXTRA®
Additional Features
THE SERACCESS® APPROACH
The automated, regular, accurate and precise glucose measurement provided by Seraccess® will empower confident treatment decisions and lead to improved clinical outcomes. Furthermore, hospitals will benefit from significant cost reduction by eliminating manual glucose measurements.
Blood Sampling
How much blood is needed per day to measure blood glucose every 15 minutes?
The Seraccess® technology only needs 20µl to 30µl of blood to perform a measurement. At a sample frequency of 15 minutes, Seraccess® administers 96 measurements every 24 hours which amounts to only 2ml to 3ml of blood per day.
Plasma Extraction
How to communicate directly with the blood?
One of the key elements of the Seraccess® technology is the proprietary silicon membrane which contains millions of pores through which pure blood plasma is extracted for an accurate, precise, and nearly delay-free blood glucose measurement.
Blood Glucose Measurement
More accurate, more precise, and nearly delay-free
The photometric method used by the Seraccess® technology provides much more accurate and precise blood glucose measurements than conventional electrochemical methods, which are subject to interfering factors such as signal drift, time delay or slow response time as well as substances such as hydroxyurea, acetaminophen, or paracetamol.
Blood Glucose Monitoring
Reliable blood glucose monitoring
An intuitive easy-to-use screen interface supports patient management. As a default, blood glucose measuring frequency is set at 15 minutes and can be adjust ed as needed. Due to this increased measuring frequency, Seraccess® AGM can lead the way towards reduced glycemic variability and improved clinical outcomes.
OPPORTUNITY
Over 200,000 beds in intensive care units in the US and Europe alone translate to a yearly potential of at least 35 million treatment days generated by about 9 million patients. An ICU bed equipped with the Seraccess® AGM durable unit will host around 100 patients per year, who will consume 200 disposable units. Overall, the value of the durable market is estimated at 1.4 bn CHF and the disposable market at 2.7 bn CHF.
Visit our Investor Relations page to contact us and learn more.
LEARN MORE
-

Seraccess® - Disruptive Technology for Monitoring Critical Blood Parameters
In its MedTech division, Securecell is proudly developing Seraccess®, an innovative technology that fully automates high-frequency collection, processing, and analysis of blood using next …
-

Seraccess® AGM / AXM Automated Glucose Monitoring for Hospital Care
Seraccess® AGM / AXM Automates Glucose measurement every 15 min. to monitor, detect and treat dysglycemia in patients using only 2-3ml of blood per day.
Disclaimer
Seraccess® is a mid-stage venture project of Securecell that offers investment opportunities to private and institutional investors.
All information and opinions contained herein have been prepared by management and its advisors and represent their assessment as of January 2022. No representation or warranty, expressed or implied, is given as to the accuracy or completeness of the contents, opinions, or projections expressed herein and no responsibility or liability is accepted. All information videos and contents do not constitute an offer to purchase securities. The website content does not constitute an invitation to buy shares under US or international law.
References:
1 I.B. Hirsch, L.M. Gaudiani: A new look at brittle diabetes. Journal of Diabetes and Its Complications 35 (2021) 107646; https://doi.org/10.1016/j.jdiacomp.2020.107646
2 Cryer, P.E., S.N. Davis, and H. Shamoon, Hypoglycemia in diabetes. Diabetes Care, 2003. 26(6): p. 1902-12.
3 Lind, M., et al., Glycemic control and excess mortality in type 1 diabetes. N Engl J Med, 2014. 371(21): p. 1972-82.
4 Cartwright A. et al. The outcome of brittle type 1 diabetes - a 20 year study. Q J Med 2011; 104:575-579
5 Flanagan D. What happens to people with “brittle” diabetes? Diabetes Digest 2011 Vol 10;4:196 https://www.pcdsociety.org/download/resource/2756; accessed Dec 16, 2021
6 https://www.webmd.com/diabetes/brittle-diabetes-all-about; accessed Dec 15, 2021
7 CDC National Diabetes Statistics Report 2020; https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf; accessed Dec 15, 2021
8 https://www.americanactionforum.org/research/understanding-the-insulin-market/; accessed Dec 15, 2021
9 https://www.bdtype1.com/difference-stable-type-1-vs-brittle, accessed Dec 20, 2021
10 Lyerla et al. Recurrent DKA results in high societal costs - a retrospective study identifying social predictors of recurrence for potential future intervention. Clin Diabetes Endocrinol (2021) 7:13; https://clindiabetesendo.biomedcentral.com/track/pdf/10.1186/s40842-021-00127-6.pdf
Related Articles on Seraccess®
SERACCESS® MILESTONE 7B
February 6, 2023Continuous automated blood sampling including glucose measurement in an animal model
SERACCESS® MILESTONE 7A
January 7, 2023Continuous automated blood sampling based on an animal model
SECURECELL PRESENTED SERACCESS AT ATTD CONFERENCE
January 5, 2022SeraMaster Version 2 – Intravenous blood glucose measurement with subjects with type 1 diabetes